
The European Medicines Agency has set up an infrastructure to support real-world monitoring of the efficacy and safety of COVID-19 vaccines and treatments.

The European Medicines Agency has set up an infrastructure to support real-world monitoring of the efficacy and safety of COVID-19 vaccines and treatments.

Stevanato Group and Colanar have signed an agreement for lab scale fill/finish capabilities to study container closure systems at Stevanato Group’s US TEC in Boston, MA.

An LIMS system enables a COVID-19 testing center for employees at their workplace.

Fujifilm Diosynth Biotechnologies’ North Carolina site will be used to manufacture Novavax’ NVX‑CoV2373 vaccine candidate for a Phase III clinical trial.

Pending approval of the Pfizer and BioNTech mRNA-based vaccine candidate against SARS-COV-2, the companies will supply the US government with an initial 100 million doses.

CMIC Bio will offer contract development and manufacturing organization services for biopharmaceutical drug substance.

Should therapies and vaccines be cheap or free in a pandemic and would that really dry up innovation?

The Acryl-EZE II works to extend Colorcon’s aqueous enteric systems by providing applications for tablets and multi-particulate dosage forms with increased enteric protection.

The project will focus on providing a faster route to clinical studies for AstraZeneca’s potential COVID-19 vaccine, AZD1222.

Catalent has unveiled plans to invest in a European center of excellence for clinical biologics formulation development and drug product fill/finish services at its facility in Limoges, France.

The UK government has secured early access to 90 million COVID-19 vaccine doses from the BioNTech/Pfizer alliance and Valneva.

Innovate UK has awarded Epsilogen with further grant funding for the development of the next generation antibody treatments for ovarian cancer.

GSK and CureVac will collaborate on mRNA-based vaccines and mAbs. Separately, the EIB and others provided CureVac with financing for development of its CVnCoV vaccine candidate and expansion of manufacturing.

Study reports immune and T-cell response from CanSino COVID-19 vaccine candidate.

Preliminary data from a German Phase I/II trial shows Pfizer/BioNTech COVID-19 vaccine candidate produces immune response.

Industry should be seeking more information from FDA on how it will restart its current inspection program.

Strong immune response by patients receiving two doses of vaccine suggests a possible treatment strategy.

Udit Batra, PhD has been named Waters’ new president and CEO, effective September 1, 2020.

The Committee for Human Medicinal Products (CHMP) of the European Medicines Agency (EMA) has issued its final opinion on measures for companies to take that will limit the presence of nitrosamines in human medicines.

Global regulatory authorities have published a report describing the aligned positions on COVID-19 vaccine development, which were agreed upon by meeting participants of the second workshop on COVID-19 vaccine development.

Industry has welcomed the launch of the Global AMR Action Fund, which aims to bring two to four new antibiotics to patients by 2030 to tackle antimicrobial resistance (AMR).

Suppliers to the bio/pharma industry are sharing news about the business operations during the global COVID-19 pandemic. To view current information about the company’s business operations, click the company name.

Dewpoint’s condensate platform works to target individual molecules and interacting groups of molecules within condensate communities to create new treatments for incurable and untreatable diseases.

Through the deal, Roche will gain co-commercialization rights to pralsetinib, Blueprint Medicine’s investigational, once-daily oral precision therapy for the treatment of RET-altered non-small cell lung cancer, medullary thyroid cancer, and other types of thyroid cancer

Suitable for bioprocessing, solubilization, and stabilization applications, the new histidine products allow for the control of endotoxin and trace metal impurities reflected with elemental impurity data in parts per billion on their specifications.
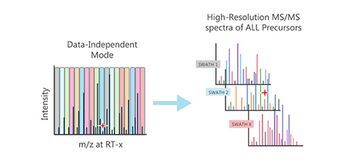
This method is expected to help bring gene therapies to market faster, safer, and cheaper.

The agency is hoping to restart performing on-site domestic inspections during the week of July 20, 2020 depending on factors such as the status of COVID-19 in the state of inspection and local rules and guidelines.

Pharmaceutical Technology spoke with Geoff Parker, co-founder of Scimcon about the impact COVID-19 is having on their business.

The agreement will accelerate production of SiO2’s plastic vials for vaccines and therapeutics for Operation Warp Speed.

Stevanato Group’s Vision Robot Unit uses AI-based machine learning capabilities for particle and cosmetic inspection of biopharmaceutical drug products.