
The way in which a suspension nasal spray product interacts with the body depends not only on the droplet size of the delivered droplet, but also on the particle size of the suspended API.

The way in which a suspension nasal spray product interacts with the body depends not only on the droplet size of the delivered droplet, but also on the particle size of the suspended API.
Nanotechnology is an important area of drug and biomedical research, and advancing nano-analysis is crucial for its further development.

Ultra high performance liquid chromatography is advantageous in a contract laboratory because it is faster, more sensitive, and relies on smaller volumes of organic solvents than HPLC.

A new book inspires readers to seek ways to apply NMR spectroscopy to their own purposes.

Michigan State University researchers have discovered a technique for viewing whole cells to gain an understanding of protein inclusion bodies.

A book about pharmaceutical analysis engages the reader with history and unexpected asides.
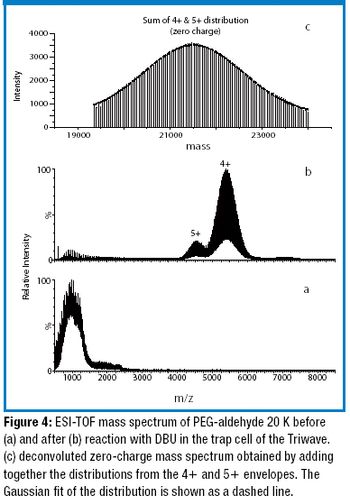
The authors developed a method to accurately measure the average molecular weight of large poly(ethylene glycols) (PEGs) using ion-mobility time-of-flight mass spectrometry coupled with gas-phase ion–molecule reactions.

USP 467 Residual Solvents will take effect on July 1, 2008. But does the industry understand these specifications-and is it prepared?
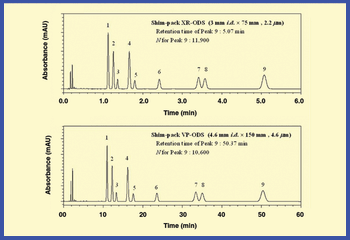
The authors review methods for reducing analysis time and increasing throughput that are reliable and maintain data integrity.
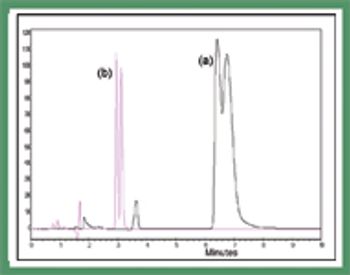
Polysaccharide-based chiral stationary phases have been developed that comprise chiral selectors immobilized on their support rather than being physically coated. These materials are completely solvent stable, thereby increasing selectivity and and enabling the development of new chiral selectors that have been too unstable in a coated form for general use.
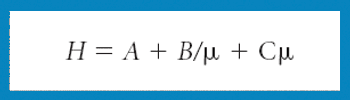
Ultrahigh pressure liquid chromatography maximizes efficiency, but, as defined by the resolution equation, the stationary phase is still a crucial consideration when attempting to resolve mixtures of compounds.

A new Raman spectroscopic method to detect magnesium stearate in powder blends and tablets is described. High-volume pharmaceutical manufacturing requires the use of lubricants to facilitate tablet ejection from compressing machines. However, lubricants may also bring a number of undesired problems that have been widely documented in pharmaceutical scientific literature. New analytical methods are needed to understand lubrication and provide process knowledge in support of FDA's process analytical technology initiative. The detection of magnesium stearate in lactose, mannitol, corn starch and other commercially important excipients is reported. The Raman spectroscopic method has a detection limit of about 0.1% (w/w) based on the 2848 cm-1 band that corresponds to the symmetric stretch of the methylene group in magnesium stearate.
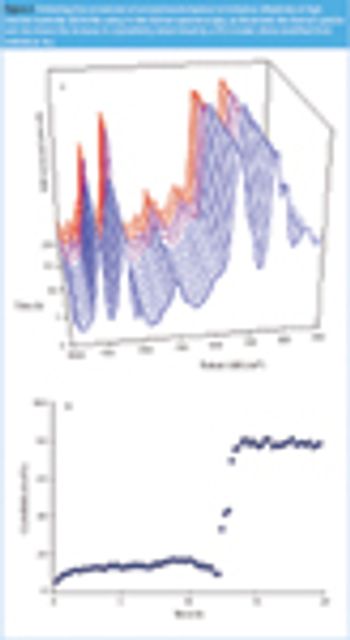
Raman spectroscopy has become a commonly used technique for physicochemical analysis that possesses many advantages over other analytical techniques. It is a very attractive characterization tool, not least because it enables measurements in water. However, very few examples of its application in an aqueous environment exist in literature. This paper provides some recent applications of Raman spectroscopy in pharmaceutical material and process characterization when water is present.
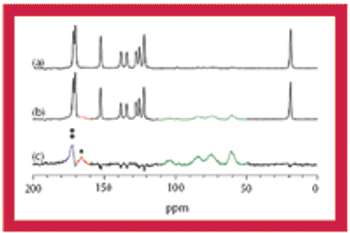
This article discusses the advantages and disadvantages of using solid-state NMR spectroscopy for the analysis of pharmaceutical solids.

The first part of this article introduced the basic features of Raman spectroscopy and presented some examples of its application in the pharmaceutical industry. This second part focusses on the technique's application as a PAT tool within the pharmaceutical manufacturing environment. FDA's PAT initiative has provided motivation to explore the application of 'new' analytical technologies to the pharmaceutical manufacturing process and Raman spectroscopy shows great promise. The strengths and weaknesses of the technique as a potential PAT tool are discussed together with some examples of how this works in practice in a pharmaceutical manufacturing environment.

Detection and identification of different polymorphic forms is, therefore, important throughout the drug development and manufacturing process.