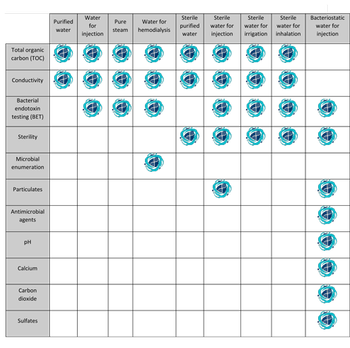
USP describes analytical and microbiology testing required for different types of pharmaceutical water.

USP describes analytical and microbiology testing required for different types of pharmaceutical water.

Increased capacity at PPD’s Richmond, VA laboratory are designed to support customers’ vaccine development programs.

Expanded storage capacity at the SGS laboratory in Mississauga, Canada increases stability testing capabilities.

The compendium provides a resource to identify and locate targeted suitable patient-derived xenografts based on specific histology and molecular properties.

New incubators, instrumentation, and staff will increase cell bioassay capabilities for EAG Laboratories.

RSSL has added new equipment for measuring the surface area of powder particles, which is important for determining the performance of excipients and APIs.

The company has secured an additional facility in Hampton, Middlesex, as part of a project to expand its UK-based operations by 15% in 2017.

SGS expands its elemental impurity testing services at its laboratory in Villeneuve-la-Garenne, France.

Analytical products for improved bio/pharmaceutical development.

Selecting the best partner contract service and fostering a successful relationship requires detailed research and effective communication.

By working together and taking a QA-based approach, manufacturers and suppliers can reduce raw material testing requirements.

SGS invested in test equipment for analyzing extractables and leachables at its New Jersey laboratory.

EAG Laboratories offers dermal absorption studies using OECD methods.
Understanding of endotoxin assays and a range of detection technologies are essential for effective testing.

Agilent’s new facility in Folsom, California includes laboratory, order fulfilment, and warehousing space.

Pressures to accelerate current and next-gen therapies are challenging traditional microbiological testing models.

Dedicated facility will address enhanced regulation of metals and impurities in pharmaceuticals.

The new facility is solely dedicated to offer extractables and leachables (E&L) testing services to the pharmaceutical and related industries.
The Claristep filtration system from Sartorius improves preparation of samples for analytical testing.

Stability testing programs should provide drug owners the information needed to establish the proper handling, shipping, and shelf-life recommendations for drug products.

Bio/pharmaceutical contract service provides continue to invest in development, facility upgrades, technological advancement, and mergers and acquisitions.

EAG Laboratories announces new company identity and intent to expand testing, analysis, and characterization capabilities across multiple markets.

Investment at SGS’s Mississauga, Canada facility provides for analysis of molecular interactions in real time.
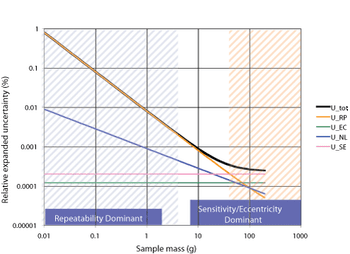
A program for calibration and routine testing of weighing instruments ensures accurate results.

Mergers and acquisitions have changed the shape of the contract services market as big players seek to build full-service capabilities.