
New instrumentation at the SGS Mississauga, Canada facility advances testing capabilities for packaging and container testing.

New instrumentation at the SGS Mississauga, Canada facility advances testing capabilities for packaging and container testing.

Upgrades to Mettler Toledo’s weighing and dosing equipment increase productivity using lean laboratory principles.

SGS Life Science Services announced that its facility in Fairfield, NJ, has been upgraded to be Biosafety Level 2 (BSL-2) compliant, according to the Centers for Disease Control and Prevention (CDC) guidelines.

WuXi's Laboratory Testing Division will be the exclusive supplier of laboratory testing services for Hong Kong-based Lee's Pharm.

With this acquisition, Source BioScience is now able to offer integrated solutions to customers, with environmentally controlled storage in combination with the required up- or downstream analytical testing services.

*This article is an opinion piece and does not necessarily represent the views of BioPharm International.

The YDP30 printer from Sartorius automatically performs thermal transfer or direct thermal printing, without the need to change settings, to meet requirements for fade-resistant printout in regulated areas, such as quality-control laboratories.

After a long wait for the new elemental impurities guidelines, the bio/pharma industry must now look ahead to implementation and take action.

Evans Analytical Group expands into the pharmaceutical/biopharmaceutical industry with acquisition of ABC Laboratories.

Keith Moore will support Metrics Contract Services as vice president of analytical services.

SGS Life Science Services expands cGMP chemistry and biotechnology testing facilities in Shanghai.

A new SGS Life Science Services laboratory outside Paris is designed for bio/pharmaceutical quality control testing.

Presenters at IVT's Microbiology Week discussed best practices and recent guidance publications for microbial control in sterile and non-sterile pharmaceutical processes.

Huber Kältemaschinenbau will be exhibiting temperature control solutions for the research laboratory and process industry at ACHEMA 2015.

Porvair has developed a 2-ml 96 round-well deep-well plate that enables scientists to use the full 2.00 ml well capacity.

This new pharmaceutical stability storage facility will enable the company to expand its analytical and formulation offerings to the pharmaceutical and biotech industries.

FDA issues a Form 483 following inspection of Impax Laboratories' Hayward, CA facility.
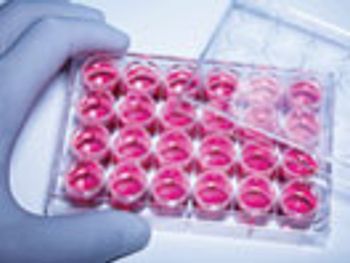
The rapid testing of biologic raw materials can lead to greater efficiency.

Stability testing and UPLC capabilities highlight expansion at Mumbai, India facility of SGS Life Science Services.

SGS Life Science Services adds analytical methods to identify amino acid impurities in bio/pharmaceutical manufacturing at facility in Germany.

SGS has invested in additional modules for its COBAS 6000 analysis system in a move to expand its biomarker analytical capabilities at its Poitiers facility in France.

The company has installed and validated new instruments for structural analysis of proteins.

EMA Recommends Suspension of 700 Drugs Tested at GVK Site GVK Biosciences argues that EMA’s recommended suspension of 700 drugs is disproportional to reported infractions. The European Medicines Agency (EMA) has issued a recommendation that 700 medicines authorized for use in the European Union (EU) should be suspended, based on concerns about how GVK Biosciences, a contract research organization in Hyderabad, India, conducted clinical studies. GVK Biosciences, in response, argued “the action is unprecedented and highly disproportional.”

Advanced analytical methods are speeding up the targeted evaluation of potential viral contaminants.

The authors present a validation study of an analytical method for the simultaneous determination of nine human cytokines in human K2-EDTA plasma.