
Sponsor companies' increasing focus on strategic outsourcing has changed the rules of the game.

Sponsor companies' increasing focus on strategic outsourcing has changed the rules of the game.

Flow chemistry and microreactors offer alternatives to traditional batch manufacturing.

Peptides and related technologies to are starting to improve production.
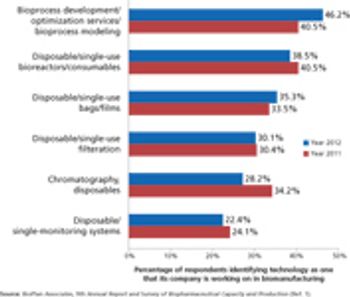
Industry wants more innovation, but can suppliers meet customer needs?

Partnerships between industry and academic medical centers are expanding to meet R&D needs.

Targeted polymeric nanoparticles are an important vehicle for controlling and targeting dosing of chemotherapeutic agents.

Advances in palladium-catalyzed hydrogenation, visible-light photocatalysis, and chemocatalyisis for heterocycles are some recent developments.

Industry, the public sector, and individuals can play an important role in creating solutions.
In Part III of a three-part article, the authors examine various degradation routes of APIs, impurities arising from interactions in formulations, metabolite impurities, various analytical methods to measure impuritie, and ways to control impurities.

With financing constrained, biotechnology firms must find ways to sustain innovation.

Contract API manufacturers and fine-chemical producers roll out capacity and service expansions.

Excipient manufacturers expand production capacity and partner to broaden their offerings.

Regulatory bodies, standard-setting organizations, and industry seek to tackle the problem of counterfeit drugs and securing the flow of pharma ingredients.

The divide between innovation and conflict of interest in medical research is not so clear.

The evolving bio/pharmaceutical business model poses risk for CMOs.

The author reviews significant changes to GMP for excipients in the forthcoming American National Standard, including a risk-based approach to excipient manufacture, why new requirements were proposed, and their potential impact to excipient manufacturers.

This article provides guidance for industry on how to comply with the pending American National Standard on excipient GMP, with a focus on risk assessment.

Taste-masking is an important consideration to ensure patient compliance.

FDA answers key questions about the October 2011 guidance on using physical–chemical identifiers in solid oral dosage products to help prevent and avoid counterfeiting.

As biopharmaceutical development and commercialization increases, companies are expanding their cold-chain capabilities.

A new class of nanoparticles hold promise for preventing premature drug release and offering greater accuracy and effectiveness in drug delivery.

A technical forum featuring Catalent Pharma Solutions, SAFC, and Neuland Laboratories.

The authors designed an upper punch with a removable punch tip to determine a tablet formulation's propensity to stick by weighing the mass of powder adhered to the punch tip.

Challenges remain, particularly for early-stage biopharmaceutical companies.

Some recent advances involve strategies for accelerating reaction discovery, approaches for inducing chirality and stereochemical analysis, and applications in nanotechnology for protein elucidation.