
Highly potent APIs (HPAPIs) represent a growing area of interest for the pharma industry. Mark Griffiths, CEO of Carbogen Amcis AG, explains why.

Highly potent APIs (HPAPIs) represent a growing area of interest for the pharma industry. Mark Griffiths, CEO of Carbogen Amcis AG, explains why.

We're all familiar with traditional pills and medicines, but how about medicated chewing gum? Marc Ribe of Cafosa Gum explains how APIs can be incorporated into a novel dosage form that can aid patient compliance.

Clarifying GMPs for excipients used as actives.

A Q&A with Brian Johnson, senior director of supply chain security at Pfizer, moderated by Patricia Van Arnum. Part of a special Ingredients issue.
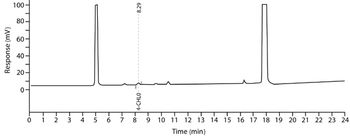
The authors provide an overview of methods for the quantitative determination of genotoxic impurities (GTIs) in active pharmaceutical ingredients.

Recently, there have been many innovations in the latest techniques and technologies in API purification. In particular, the trend to single-use systems has had a significant impact on processes.
A perspective from Pfizer on the lessons from small-molecule manufacturing that can be applied to biomanufacturing.

The author examines sample-preparations methods used in inductively coupled plasma–optimal emission spectroscopy for four test metals.

Achieving a consistent level of quality control could greatly reduce waste and save money for the pharmaceutical industry. But why has talk about Six Sigma died down at a time when it could be of great benefit?

The Society for Chemical Manufacturers & Affiliates commented on EPA's modifications to the Inventory Update Reporting rule, also broadly know as the Chemical Data Reporting rule. EPA issued the final rule earlier this month.

Merck & Co. announced last week that it plans to reduce its global workforce, as measured by year-end 2009 levels, by an additional 12–13% by 2015. The company made the announcement as part of its second-quarter earnings release, which the company issued on July 29, 2011.

Contract manufacturing organizations throughout Asia are increasing their capabilities to meet market demand and attract foreign investment and partnerships.

O-arylation and O-alkylation, a one-pot protein synthesis, a combined approach in continued and chemocatalysis, and green-chemistry applications are the target of some recent advances in API synthesis.
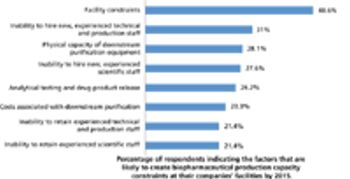
China rises to the top as a destination for international outsourcing.

A path to personalized medicines creates a new paradigm for development and manufacturing.
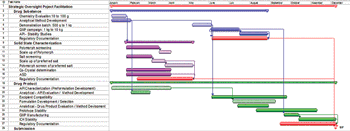
The article examines a cross-functional supplier integration model to facilitate project management.

Pfizer's Experience with QbD. This article is part of a special issue on Outsourcing.
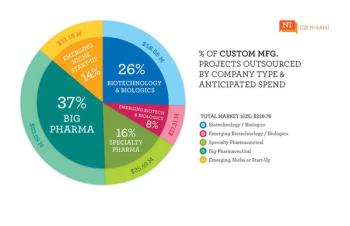
The article examines the drivers of customer perception of contract service providers of pharmaceutical development and manufacturing.

An Industry Roundtable Moderated by Patricia Van Arnum and Rich Whitworh. Contract service and technology providers share their perspectives on the influence of quality by design in the expectations between sponsor companies and outsourcing providers.

The DHS announced it has revised tiering assignments for several chemical facilities covered under DHS's Chemical Facility Anti-Terrorism Standards program, which requires chemical companies to develop and implement specific security plans for their facilities.

SOCMA has issued its support this week of the passage of pending free-trade agreements with South Korea, Panama, and Colombia by two Congressional committees.

FDA, in cooperation with IPEC, is building a spectral library of excipients to detect improper ingredients within a drug product on site.

The performance of biotechnology venture capital and investment is lackluster at best.

The solid form of an API plays a crucial role in drug quality, and advancing methods for screening, detection, and characterization is key.

Member states in the EU are working to implement the newly passed Falsified Medicines Directive.