
Company and People Notes: Pfizer Completes King Tender Offer; Cook Pharmica Names VP of R&D and CSO; and More

Company and People Notes: Pfizer Completes King Tender Offer; Cook Pharmica Names VP of R&D and CSO; and More

Follow-ons were all the rage at this year's JP Morgan Healthcare Conference.
Will 2011 be a more promising year for new molecular entities? A review of Big Pharma's late-stage pipeline shows what might lie ahead.

As biologic-drug patents move toward expiration in the US, Indian firms with experience in the follow-on biologics arena are eager to partner with global manufacturers and secure their place in the growing biosimilars market.

Q&A with Magnetrol International's Dan Klees

As the industry prepares for Informex, the trade show of custom and batch manufacturers in Charlotte, North Carolina, a roundup of key recent developments.

The article examines pharmaceutical market growth, company positioning, and the innovation potential in emerging markets. Read this and other preferred organization articles in this special issue.

Pharmaceutical-industry executives from Merck & Co., Pfizer, and Covidien share their perspectives on their expectations and evaluation in the preferred-provider relationship. Read this and other preferred organization articles in this special issue.

Novartis Acquires Genoptix; Lilly Exec Becomes Savient CEO; and More.

Merck Teams with Parexel; Roche CFO to Retire; and More

During the past few years, we've seen a growing interest in direct oral application products, evidenced by the large amount of products already available on the market.

Orally disintegrating tablets (ODTs) are growing in popularity and their market value could exceed $13 billion in sales by 2015, based on current global growth trends.

Iain Moore explains what progress has been made so far regarding EU plans to regulate excipients.

Biologics are large molecular weight molecules primarily formulated for parenteral administration; however, there are some smaller biomolecules that have been formulated for oral use.

Excipients should be regulated to manage risk. Although, excipients are an integral part of a finished pharmaceutical product, the issue of regulation is complex.

Gilead Sciences to Acquire Arresto Biosciences; Sartorius Appoints Head of Lab Business; and More.

US Senator Amy Klobuchar (D-MN) urged the US Food and Drug Administration and the pharmaceutical industry to address what she called an "unprecedented" shortage of prescription drugs, especially for chemotherapy.
Contract manufacturers strengthen their toolboxes and partnerships as they navigate the changing drug-development model.

The European Commission and Medicines Agency seem to be moving in advance of their ICH partners to update standards.

Expert and implementation working groups harmonize more guidelines and move Q11 forward.
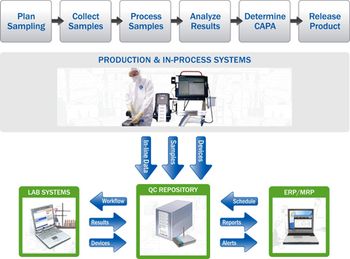
The authors discuss the role of quality-control automation in providing better data, enhanced compliance, and potentially faster release times.

The authors group key actions to consider when conducting a product recall and discuss how to execute them carefully and thoroughly.

Capable of great works, pharma as a whole still yields to the lesser angels of its nature.

A Q&A with Spectrum Laboratories President Tony MacDonald.

Holding product and supply-chain security to the highest standards is crucial for the future.