Process chemists employ a variety of approaches to improve yield, purity, and stereoselectivity.

Process chemists employ a variety of approaches to improve yield, purity, and stereoselectivity.
As 2012 comes to a close, Pfizer leads among Big Pharma companies for FDA approvals of new molecular entities and biologics.

The promise of antibody drug conjugates is creating a network of partners among large pharma companies and specialized players.

Developing analytical methods and performing related testing is crucial for ensuring the quality of a pharmaceutical product.

Recent research on elucidating the structure and sequence of proteins involves examining the effect of microgravity on protein crystallization and a computational model for protein elucidation.

The authors examine the use of various grades of direct-compression mannitol in direct-compression tableting process to evaluate the content uniformity of micronized APIs and excipients in a solid-dosage formulation.

Particle-engineering technologies, such as crystal design for crystallization and producting cocrystals, particle-size reduction, and amorphous solid dispersions, help to optimize delivery of a drug.

The long-awaited patent cliff that has loomed in the pharmaceutical industry for years has arrived in earnest in 2012, with more than $40 billion in 2011 brand sales facing loss of exclusivity.

Bristol-Myers Squibb has initiated a voluntary recall of 10 lots of BiCNU (carmustine for injection) previously manufactured by Ben Venue Laboratories, a former, third-party contract manufacturer for the company, to the user level.

A review of taints and odors in the pharmaceutical and consumer healthcare industries.

Industry and academia advance novel approaches for achieving enanioselectivity.
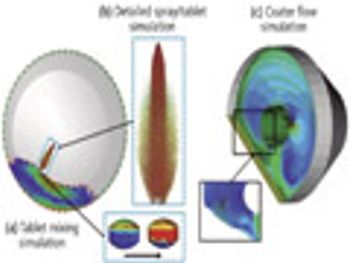
The authors investigate the tablet-coating process using a combination of different simulation techniques.

Pfizer has two manufacturing facilities in Germany for high-potency manufacturing, respectively in Freiburg and Illertissen. Pharmaceutical Technology's Executive Editor Patricia Van Arnum visited the facilities and spoke to the company about the design and operation of these facilities.
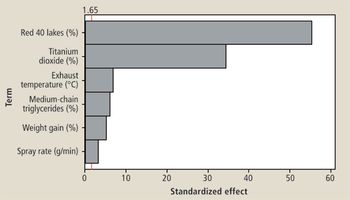
The authors describe a QbD study that was performed to optimize a coating system.
The authors explain chemical transformations that are achievable through certain biocatalytic routes.

Policymakers must balance fundamental issues involving access to medicines and pricing.

An industry roundtable representing Metrics, Cambrex, Carbogen Amcis, Euticals, Ferro Pfanstiehl, and SAFC.

The author discusses the key provisions of GDUFA as they relate to the pharmaceutical supply chain, including parity of inspections between domestic and foreign sites for both finished dosage forms and APIs of generic drugs.

The author discusses strategies for preventing cargo theft.

Approaches to scaling up API syntheses center on ways to optimize process conditions and operability.
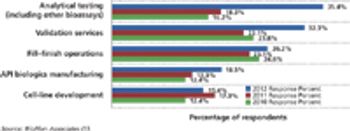
Budgets for biopharmaceutical activities are gaining in select functional areas except outsourcing.
Enhancing bioavailability can be achieved through hot-melt extrusion or spray drying. Patricia Van Arnum interviews Bend Research to find out more about when to use each technique.
The manufacturing capacity-sharing model in biologics and Merck & Co. and MedImmune ushers in a new paradigm of "co-opetition".
An examination of the current and projected market for biosimilars, development costs for biosimilars compared with small-molecule generic drug, and partnerships in biosimilars.

Flow chemistry and microreactors offer an alternative to traditional batch manufacturing.