
USP seeks input from stakeholders on new and revised standards to mitigate extractables and leachables in plastic packaging systems.

USP seeks input from stakeholders on new and revised standards to mitigate extractables and leachables in plastic packaging systems.

Industry experts discuss the importance of characterization studies during biosimilars development and related analytical methods.

Following passage in the California Assembly, the California State Senate passes legislation to specify requirements for dispensing biosimilars.

Industry experts discuss the requirements and challenges involved in getting a biosimilar product from bench to launch.
Extensive comparability testing is required to ensure that biosimilars have comparable profiles to their reference products.

EMA's revised guideline on biosimilars containing biotechnology-derived proteins is published for public consultation.

An overview of the latest regulatory developments for malaria drugs, biosimilars, and global standards.

BioOutsource, a contract testing services provider to the biopharmaceutical industry, has opened a new facility in Cambridge, Massachusetts. The facility marks the company's first North American offices, which were opened in response to increasing demand for the company's bioanalytical and biosafety services, particularly in the field of biologic and biosimilar characterisation.

Teva and Lonza have announced that their joint venture will continue to develop, manufacture and market affordable, efficacious and safe biosimilars.

Sound policies are needed to govern the substitution of interchangeable biologics.

Latest news about compound pharmacies, biosimilars, prescription drug purchases, and other regulatory topics.

Merck and Samsung Bioepis have formed an agreement to develop and commercialize multiple prespecified and undisclosed biosimilar candidates.

FDA has released a list of more than 50 guidance documents planned for 2013.

Innovation resulting in improved productivity continues unabated and is a primary driver for many of the current biopharmaceutical trends.
The European Medicines Agency has added granularity to its biosimilars approval pathway by releasing a guideline on biosimilar monoclonal antibodies (mAbs).
An examination of the current and projected market for biosimilars, development costs for biosimilars compared with small-molecule generic drug, and partnerships in biosimilars.

Dr. Reddy's Laboratories and Merck Serono, a division of Merck KGaA, have partnered to codevelop a portfolio of biosimilar compounds in oncology.
Extensive physicochemical characterization of the innovator product and the proposed biosimilar provides the foundation for demonstrating biosimilarity.

Does global development have to entail multiple comparability studies?

Poland's government aims to make the Eastern European country a biotech powerhouse.

On Mar. 29, 2012, the Generic Pharmaceutical Association reiterated its call for Congress to move forward with user-fee proposals for generic drugs and biosimilar products.

Has the long-awaited guidance answered all of the industry's questions?

On Dec. 6, 2011, FDA announced that a public meeting will be held on Dec. 16, 2011 to discuss recommendations for a user fee program for biosimilar biological products for fiscal years 2013–2017.
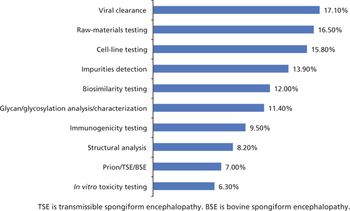
Biosimilar manufacturers need better expression systems and analytical tools to compete.

Since the passage of the BPCI Act in 2009, manufacturers have been waiting for guidance from FDA on what that approval process will look like.