
The Cell Therapy Catapult, University of Birmingham, and Cancer Research Technology collaborate on CAR-T cell immuno-oncology therapy development.

The Cell Therapy Catapult, University of Birmingham, and Cancer Research Technology collaborate on CAR-T cell immuno-oncology therapy development.

TxCell signs strategic agreement with MaSTherCell for European manufacturing of its cell therapy products.

A study backed by the Centre of Regenerative Medicine found that laminins are crucial cell components that could help aid the commercial production of stem cell therapies.

Cell Therapy Catapult will provide manufacturing scale-up services to enable Asterias’ future clinical trials and commercial supply for Asterias’ allogeneic dendritic cell immunotherapy, AST-VAC2.

The Cell History File is designed as a “cell passport” for developers and manufacturers of tissue- and cell-based medicinal products.

The company is in discussions with Europe’s Adaptive Pathways Group on clinical trials that would evaluate its PLX cells for the treatment of specific diseases.

apceth has completed a major GMP inspection for two medicinal products. The two cell and gene therapy products are now included in the company’s GMP manufacturing license according to §13 of the German Medicines Act (AMG).

Lonza’s planned facility will be used to develop and manufacture viral gene therapies and virally modified cell therapies

Pluristem Therapeutics announces that Japan’s Pharmaceuticals and Medical Devices Agency agreed with its methods for the manufacture of PLX-PAD cells.

INTERPHEX 2015 is under way and Pharmaceutical Technology and BioPharm International are in the middle of the action!

Sessions address cell therapies, tableting, continuous processes, serialization, and more.

The Berlin-Buch facility will begin manufacture of the company’s immune cell therapy.
Whether outsourcing or developing cell therapies in-house, success demands a focus on quality, cost of goods, and sustainability from the start.

Better equipment and automated processes will be key. So, too, will the right way of approaching outsourcing relationships.

On December 8, Pfizer announced that it will establish a research program in gene therapy, and collaborate with Spark Therapeutics in Philadelphia, to develop potential gene therapy treatments for hemophilia.
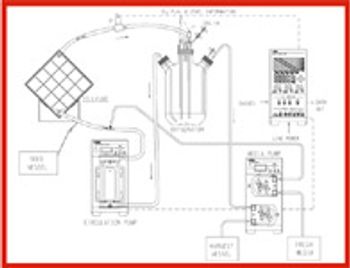
Creating a kinder, gentler manufacturing process that doesn't kill the product is the goal of process developers doing large-scale cell culture for cell therapy.