
Ginkgo Bioworks has acquired StrideBio's AAV capsid discovery and engineering platform and has formed a partnership with WARF for development of next-gen cell therapies.

Ginkgo Bioworks has acquired StrideBio's AAV capsid discovery and engineering platform and has formed a partnership with WARF for development of next-gen cell therapies.

Forecyte Bio has opened a new GMP facility in Shanghai just two months after its sister site in the United States.

Sartorius’ acquisition of Polyplus is designed to strengthen its cell and gene therapy capabilities.

Decreasing vein to vein time saves lives.

The combined solutions are currently available on the market.

CBM’s plasmid manufacturing offering is designed to provide phase-appropriate plasmids on demand for companies working on cell and gene therapies.
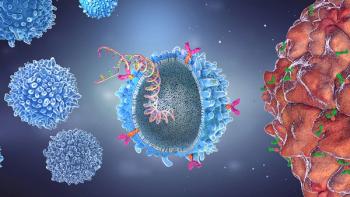
In vivo CAR-T gene therapies could resolve challenges faced by autologous and allogeneic treatments.
TreeFrog Therapeutics is leaping ahead in cell therapies through resources such as new technologies and investor partnerships.

CellVax Therapeutics has selected Theragent, a new CDMO, to manufacture clinical trial material for a new Phase II prostate cancer immunotherapy drug candidate.

The newly formed partnership between CSafe and BioLife will expand cold chain supply chain solutions for the cell and gene therapy market.

Vibha Jawa, executive director at Bristol Myers Squibb, and Luke Schenck, principal scientist at Merck & Com, discuss cell & gene therapies and co-processed APIs respectively.

Production of viral vectors requires a holistic view of the product, including its manufacturing process and its ultimate end use.

Whether biologic manufacturers decide to outsource or develop products internally, the quality of a CDMO partnership is critical to success, especially for cell and gene therapy products.

Biopharma focuses on streamlining biomanufacturing and supply chain issues to drive uptake of cell and gene therapies.

Experts from Umoja Biopharma dive into cell therapy development in this episode of the Drug Solutions Podcast, including the greatest advancements in cell therapy to date, areas for improvement, the biggest trends in cell therapy development, and retention as a pain point.

Charles River Laboratories and Cure AP-4 will collaborate on gene therapy manufacturing for AP-4 hereditary spastic paraplegia.

Forecyte Bio and Cytiva will team up to accelerate the development and manufacturing of cell and gene therapies.

Pfizer and Touchlight have signed a patent license agreement for Pfizer to use Touchlight’s doggybone DNA (dbDNA) in the manufacture of mRNA vaccines, therapeutics, and gene therapies.
In this exclusive Drug Digest video, experts from Roche and MilliporeSigma divulge factors that could influence an organization to pursue specific biomolecules for development.

Inceptor Bio and the University of Minnesota aim to build a novel iPSC platform to accelerate cell therapy drug development.
Precompetitive consortiums seek solutions to industry-wide challenges.

AGC Biologics is investing in viral vector suspension technology at its new Longmont, Colo., facility.

Through a collaboration, Avantor and Cytovance Biologics will accelerate plasmid optimization and sourcing services for viral vectors and mRNA-based vaccines and therapeutics.
In this episode of Drug Digest, Pharmaceutical Technology editors, Felicity Thomas and Feliza Mirasol, examine the topic of emerging therapies in more detail, covering subjects such as the challenges of scale, the potential benefits of drug delivery innovation, importance of early analytical studies, the evolution of the regulatory landscape, and differences between regional regulatory requirements.

In this episode of the Drug Solutions Podcast, Feliza Mirasol, Pharmaceutical Technology’s science editor, discusses technologies enabling biologics and emerging therapies manufacturing and development with Barry Holtz, PhD, chief scientific officer of Phylloceuticals, and Professor Yaakov Nahmias, founder and chief scientific officer, Tissue Dynamics and founder and president, Future Meat Technologies.