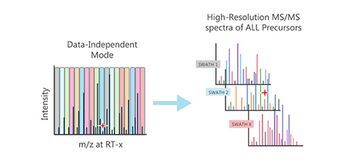
This method is expected to help bring gene therapies to market faster, safer, and cheaper.

This method is expected to help bring gene therapies to market faster, safer, and cheaper.
RNA is easier to manipulate than DNA but challenging to deliver to the right cells.

The site can now support customized product and bioprocess development and custom cell and gene therapy reagent manufacturing.

The companies are entering into a license agreement to provide CSL Behring with exclusive global rights to etranacogene dezaparvovec, uniQure’s investigational gene therapy for patients with hemophilia B.

The company will invest $75 million into its Canton, MA, facility to expand its viral vector, gene therapy, and contract development and manufacturing capabilities.

Previous investments set a foundation for later efficiency improvements.

The VIA Capsule from Cytiva is a liquid nitrogen-free cryogenic shipment system designed to transport cell therapies.

Ori Biotech is partnering with manufacturers and materials suppliers to develop an automated system for cell and gene therapy manufacturing.

Synthetic depth filters are a suitable option to provide a high-performance and scalable single-step clarification process.

The company will invest $180 million to construct a new 290,000-ft2 facility in Plainville, MA.

Hitachi Chemical Advanced Therapeutics Solutions and apceth Biopharma entered into long-term development and manufacturing services agreements for the clinical and commercial supply of multiple bluebird bio therapies.

The center will be located at NJIT’s Life Sciences and Engineering center and will feature two good manufacturing practices suites that are expected to be completed and operational during the summer of 2020.

The new cell-line producing platform enables fully scalable production of high-performance adeno-associated virus vectors.

The company will build an additional commercial-scale, contract manufacturing facility for viral vectors and gene therapies near its existing site in Carlsbad, CA.

Daiichi Sankyo has entered into a partnership with Ultragenyx Pharmaceutical for the use of Ultragenyx’s proprietary AAV-based gene-therapy manufacturing technology.

The new patented process results in uniform, scalable production and the ability to deliver cell cargo similar to natural exosomes/extracellular vesicles.

Thermo Fisher is focused on investing to expand three specific areas of demand: biologics, cell and gene therapy, and drug product development and commercial capabilities.

In January 2020, the agency finalized six clinical development and manufacturing guidance documents and drafted new guidance on what would qualify new gene therapies as orphan drugs.

Tackling process development early on can better optimize manufacturing processes for emerging therapies.
The growing interest in developing cell and gene therapies has prompted industry investment to grow manufacturing capacity.

The last year has seen intense investment activity into raising cell and gene therapy manufacturing capacity.

The 135,000 square foot facility will be constructed over 18 months and is expected to be operational in 2021.

New funding brings competitors, and a leading healthcare products distributor, into the standardization effort.

The new facility includes six classified environment rooms with space to expand.

Contract partners must help innovators, especially smaller and virtual companies, consider manufacturability as early as possible in development. This requires focusing on technical and operational performance, as well as cost.