
PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the December 2009 edition from Sepha and AdvantaPure.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the December 2009 edition from Sepha and AdvantaPure.

The US Pharmacopeial Convention posted a proposed revised standard for what should and should not appear on the ferrules and cap overseals of medication vials.

Also, Merck & Co. extends collaboration with Idera Pharmaceuticals; Pfizer establishes R&D center in China; more...

Particularly in a more connected world, individual contributions can make a difference.

Dumbed-down presentations and poor speaker selections are destroying a valuable industry tool.

With so many healthcare and pharmacy websites, consumers could use the agency's nod of support.

Company and People Notes: Merck KGaA to build R&D hub in Beijing; Depomed appoints VP of operations; more...

Company and People Notes: Genzyme provides updates on enzyme replacement products and FDA complete response letter; Watson appoints VP of global operations; more...

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the November 2009 edition from Enercon and Schreiner.

Company and People Notes: 3M forms agreement with VaxInnate; TRIN Pharma appoints CEO; More...

Company and People Notes: sanofi aventis forms pact with Micromet; Laureate Pharma adds members to its business team.

While Congress debates hundreds of healthcare plan proposals, perhaps we, the public, can get in the game too.

FDA's Edwin Rivera-Martinez on dodging supply-chain challenges.

Editors' Picks of Pharmaceutical Science & Technology Innovations
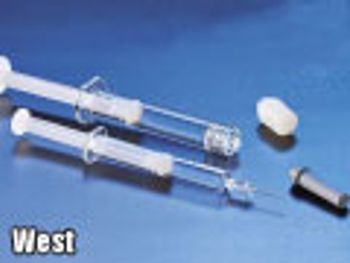
The authors describe the ways in which plastic prefilled syringes can be an alternative that provides consistent performance, protects drugs prone to degradation, and enhances patient safety.

According to a survey conducted by Pharmaceutical Technology Europe (PTE), almost 50% of you believe that cost is the biggest limiting factor to innovation in the pharmaceutical packaging industry.

Following years of development, DataMatrix codes are now a viable labelling solution for pharma manufacturers.

Michael Rubenstein, Chief Growth and Innovation Officer at Alcan Packaging, talks about applying a philosophy of sustainability to the pharmaceutical packaging supply chain.

In 2010, Braille embossing will be required on all European pharmaceutical packages. Is the industry ready?

What trends have you witnessed in the pharmaceutical packaging industry in the last 2 years?

Also, Astellas and Medivation sign development pact; WuXi PharmaTech appoints VP of business development, more...

New barcoding technologies are helping to aid product traceability and fight counterfeits. Mark Beauchamp of Citizen Systems Europe explains how.

The US Food and Drug Administration issued a guidance for industry and review staff titled, Labeling for Human Prescription Drug and Biological Products-Determining Established Pharmacologic Class for Use in the Highlights of Prescribing Information.

Company and People Notes: SurModics forms agreement with Roche and Genentech; Hospira names Daphne Jones senior VP and chief information officer; more...

Company and People Notes: Bayer Schering Pharma forms collaboration with Compugen; Takeda San Francisco appoints VP of process science; more...