
Pharmapack Europe 2021 has been rescheduled after a careful review by the event organizers with key stakeholders.

Pharmapack Europe 2021 has been rescheduled after a careful review by the event organizers with key stakeholders.

Data and software identify optimum pharmaceutical packaging choices for the required shelf-life.

The new Uhlmann bottle line uses Cremer technology for 100% counting accuracy and an inspection and rejection system for all critical quality parameters.

The new Uhlmann bottle line uses Cremer technology for 100% counting accuracy and an inspection and rejection system for all critical quality parameters.

The refurbishment provides enhanced glass melting power, improved glass distribution, and the production of transparent and amber type I glass bottles to meet the increase in demand for glass vials for vaccines and life-saving medicines.

The expansions included enhancing high-speed automatic syringe assembly and labeling, vial labeling and cartoning, and auto-injector assembly.

New options for pharmaceutical tubes enhance functionality, sustainability, and brand identity.

Corning will use BARDA funding to expand US manufacturing capacity for pharmaceutical tubing and vials made from Valor glass.

SiO2 Materials Science has received $143 million from the US government to accelerate capacity scale-up of its advanced primary packaging platform.

The VIA Capsule from Cytiva is a liquid nitrogen-free cryogenic shipment system designed to transport cell therapies.

A new clinical and commercial partnership expands Experic’s presence while providing PCI with advanced powder-filling equipment.

Advances in intelligent technologies for pharmaceutical packaging improve online productivity, authenticate product, and boost patient adherence.

RFID, NFC, and barcodes on pharmaceutical containers enhance patient safety and adherence.

The International Safe Transit Association’s Pharma Committee published a guideline on the passive thermal packaging qualification process.

More sustainable and functional packaging protects temperature-sensitive drugs.

Labeling equipment for primary and secondary packaging and a new label printer improve speed and quality.

Understanding of scale-up parameters and use of process analytical technology are important to meet demand for larger batch sizes.

The acquisition will bring four new GMP-compliant facilities to the PCI network in the United States, Germany, and Canada.

The Brand Security Platform from ACG Inspection uses blockchain, the Internet of Things, and artificial intelligence technologies to track packages from manufacturer to consumer.

The companies plan to provide characterization testing of container-closure systems for the biopharma industry.

The company announced it will be unveiling a new name, Jones Healthcare Group, and a new corporate logo as it celebrates 100 years in the health and wellness markets.
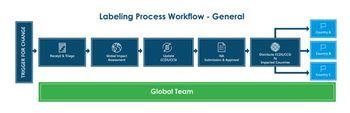
Tracking and implementing label changes are crucial to the lifecycle of a marketed drug product.

Pharmaceutical companies work toward a circular economy by using sustainable packaging.

As a new decade has begun, this editorial reviews some of the biggest, brightest, and boldest happenings from the industry over the past 10 years.
Regulatory mandates, niche diseases, and patient-centric solutions have all impacted pharma packaging over the years and are expected to help shape the future of the sector.