
Pharma companies could benefit from the lessons learned in this fall's financial crisis.

Pharma companies could benefit from the lessons learned in this fall's financial crisis.

Production problems come in all shapes, sizes, and ... species.

IPEC Chairman Dave Schoneker discusses current efforts toward facilitating regulatory reviews of new excipients.
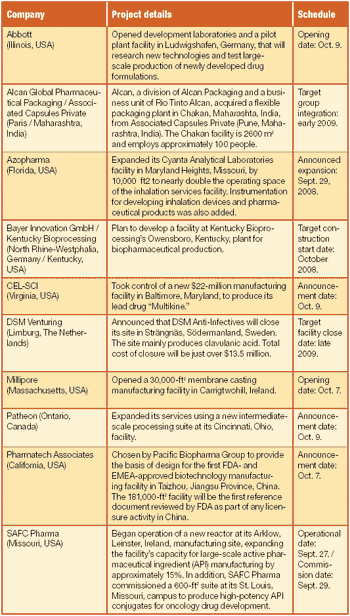


Scott Sutton discusses the current state of USP ‹1117› and USP's plans for future revisions.

During the past two decades, regulations have evolved in both Europe and the US to accommodate the technological developments in the pharmaceutical, biotechnology and medical device industries.

The US Government Accountability Office (GAO) released a report last week that examines and provides recommendations for the US Food and Drug Administration's foreign inspection process, including the agency's data management, inspection frequency, and oversight of problems identified during inspections.

The US Food and Drug Administration has awarded the National Institute for Pharmaceutical Technology and Education (NIPTE), a not-for-profit organization comprising 11 universities, a $1.19 million contract to develop quality by design (QbD) guidance elements for design space and scale-up of unit operations.

Pfizer and Lilly recently resolved issues related to their marketing practices for certain products.

The US Food and Drug Administration released a draft guidance that reviews the agency's plan to offer priority-review vouchers to companies developing new treatments for neglected tropical diseases.

Rep. John D. Dingell (D-MI), chairman of the US House of Representatives Committee on Energy and Commerce, and Rep. Bart Stupak (D-MI), chairman of the Oversight and Investigations Subcommittee, sent letters to the US Food and Drug Administration, Shaw Science Partners, and EthicAd to request information about a new FDA website.

According to a July 11, 2008 memorandum posted by the Center for Biologics Evaluation and Research, starting Oct. 15, Health Level 7 structured product labeling in XML (extensible markup language) will be the only acceptable presentation in electronic format for the submission of content of labeling that CBER can process, review, and archive.

FDA has completed its labeling updates to fluoroquinolone antimicrobial drugs.

During the past several months, Pharmaceutical Technology has been covering the US Food and Drug Administration's rulemaking on over-the-counter (OTC) cough and cold medications for children.

At a public meeting, the Medicare Payment Advisory Commission (MedPAC) discussed its recommendation that Congress establish a national database to publicly reveal financial relationships between physicians and the pharmaceutical industry.

John Dingell (D-MI), chairman of the US House Committee on Energy and Commerce and Bart Stupak (D-MI), chairman of that committee's Subcomittee on Oversight and Investigations, directed a letter to US Food and Drug Administration Commissioner Andrew C. von Eschenbach to request further information regarding FDA's process for inspecting manufacturing facilities of the generic drug manufacturer Actavis following several product recalls by the company.

The lack of appropriate financial resources for the US Food and Drug Administration's growing global agenda has been a pertinent topic of late?and now appropriate staffing has become a large concern.

A bill that would require country-of-origin labeling for active and inactive ingredients for all prescription and over-the-counter pharmaceuticals was introduced in the US Senate last week.

The US Food and Drug Administration?s Center for Drug Evaluation and Research (CDER) held a public meeting on Oct. 2 to discuss over-the-counter (OTC) cough and cold medications for pediatric use.

Rep. John D. Dingell (D-MI), chairman of the US House of Representatives Committee on Energy and Commerce, and Rep. Bart Stupak (D-MI), the chairman of the Oversight and Investigations Subcommittee, sent a letter to the US Department of Health and Human Services (HHS), questioning the US Food and Drug Administration?s use of agency resources to hire an outside public-relations firm to create a positive public image of the agency.

The European Fine Chemicals Group (EFCG) and the International Pharmaceutical Excipents Council of Europe (IPEC Europe) announced the formation of a European Pharmaceutical Excipients Certification Project to develop advocacy and stakeholder management in Europe and to give advice to two European working teams as part of an effort to develop a certification program for manufacturers and distributors of pharmaceutical excipients.
A recent report shows a decline in NASs and a rise in NME applications in 2007.

Planning ahead will ensure successful efforts to improve packaging and packaging operations.

Advancements add yet another challenge for industry's already overextended regulatory body.