
With most deadlines missed for reviewing each abbreviated new drug application (ANDA) it receives, the US Food and Drug Administration is likely to take advice from the US Department of Health and Human Services (HHS) on how to speed up its process.

With most deadlines missed for reviewing each abbreviated new drug application (ANDA) it receives, the US Food and Drug Administration is likely to take advice from the US Department of Health and Human Services (HHS) on how to speed up its process.

Effective July 1, 2008, the Office of Generic Drugs (OGD) will require abbreviated new drug applications (ANDAs) to include data that demonstrate the manufacturer's control of residual solvents.

The US Food and Drug Administration and the European Medicines Agency will collaborate in efforts that would allow drug companies to submit results of seven new drug-safety tests.

Senator Sherrod Brown (D-Ohio), member of the Senate Health, Education and Labor Pensions (HELP) Committee, is requesting additional information from the US Food and Drug Administration and the pharmaceutical industry, concerning the regulation and practice of outsourcing.

The number of clinical-trial investigators overstepping their bounds has grown in the past few years. And, says Congress, the US Food and Drug Administration is taking too long to wrap up unresolved cases.

The European Chemicals Agency (ECHA), the European Union regulatory body overseeing the implementation of the REACH (Registration, Evaluation, and Restriction of Chemicals) regulation, began full operations on June 1.

In an assessment report about medicinal products containing heparin, the European Medicines Agency (EMEA)'s Committee for Medicinal Products for Human Use (CHMP) said it could not draw firm conclusions about the level of risk associated with unfractionated heparins (UFH) contaminated with oversulfated chondroitin sulfate (OSCS). Nevertheless, CHMP recommended that contaminated lots be withdrawn completely.

The steering committee of the International Conference on Harmonization and its expert working groups adopted Quality Guideline Q10 "Quality Systems" last week at a meeting in Portland, Oregon.

Also, Pall plans expansion in South America, Anthony Clarke joins Alexza Pharmaceuticals, more...

The US Food and Drug Administration issued a new guidance Monday on indexing structured product labeling. The Center for Biologics Evaluation and Research and the Center for Drug Evaluation and Research will begin indexing structured product labeling in the product labeling for human drug and biologic products.

The transatlantic cooperation of the European Commission, the European Medicines Agency, and the US Food and Drug Administration was recognized at the Second Meeting of the Transatlantic Economic Council, held mid May in Brussels. The TEC is tasked with overseeing and accelerating government-to-government cooperation to advance economic integration between the United States and the European Union.

The California Senate passed SB 1096 on May 29, voting to amend the state?s Confidentiality of Medical Information Act to allow pharmacies to provide third parties with patient information for the purpose of mailing prescription refill reminders and drug information directly to patients.

The US Food and Drug Administration advised patients, caregivers, and healthcare professionals to switch to hydrofluoroalkane-propelled albuterol inhalers now because chlorofluorocarbon-propelled inhalers will not be available in the US after Dec. 31, 2008.

As part of a larger effort to address drug counterfeiting, the European Commission is seeking to tighten manufacturing and related supply-chain requirements for active pharmaceutical ingredients.

Comparative-effectiveness analysis aims to promote appropriate pharmaceutical spending.

Customers complaining lead to some serious explaining.

Brief pharmaceutical news items for June 2008.

The author provides an overview of key regulatory issues facing companies seeking to market their biopharmaceutical agents globally.

Risk management, and its benefits for patients, plays a big role at the PDA Annual Meeting.

With rising drug deveopment costs and burdensome clinical trials, Indian-based firms are transferring their research departments to other entities in hopes of saving cash, mitigating risk, and ultimately, buying back the rewards.

Letting the public inside the drug development process may increase their faith in what we do.
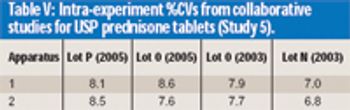
The authors demonstrate that anecdotal reports of prednisone tablet variability are inaccurate.

The good, the bad, and the ugly about direct-to-consumer advertising.

The US Senate approved a measure (HR 2642) that would provide the US Food and Drug Administration with $275 million in additional funding under a supplemental appropriations bill. The measure now goes before the House.

In a white paper published today, the US Food and Drug Administration described its Sentinel Initiative to create an electronic safety system that tracks drug performance.