Protein characterization is a critical part of drug development, but as there are still limitations with available techniques, industry needs to look at technological advances to meet the specific requirements of complex molecule characterization.

Protein characterization is a critical part of drug development, but as there are still limitations with available techniques, industry needs to look at technological advances to meet the specific requirements of complex molecule characterization.

Senior managers of OTC drug companies are on a learning curve, as FDA warning letters cite insufficient understanding of cGMPs and inadequate responses to prior 483s.

FDA, Health and Human Services, and the Trump Administration back cheaper foreign drugs to cut pharma costs.

A one-size-fits-all strategy is not the best approach for the development of a chemistry, manufacturing, and controls program.

Now that the first genetically modified cell therapies are being manufactured, the industry must move beyond “whatever works” to meet growing demand.

Performing a compliance gap assessment and focusing on six key factors in your facility’s process definition and controls can help your facility pass its next FDA inspection with flying colors.

Mass-produce cell and gene therapies presents the biopharma industry with a unique set of challenges.

Daiichi Sankyo Europe has revealed the results of a Phase III study assessing the efficacy, safety, and tolerability of bempedoic acid/ezetimibe fixed-dose combination (FDC) tablet.

CHMP and CMDh have recommended that all marketing authorization holders (MAHs) of medicines containing liposomal drug delivery systems change the medicine name to avoid medication errors.

Orion Corporation announced it has received approval from the US Food and Drug Administration for darolutamide, a new treatment for men with non-metastatic castration-resistant prostate cancer (nmCRPC).

Bayer is voluntarily recalling two lots of Kogenate FS antihemophilic factor (recombinant) 2000 IU vials because they contain the incorrect product.

EMA has issued a recommendation that the multiple sclerosis drug, Gilenya (fingolimod), not be used in women who are pregnant.
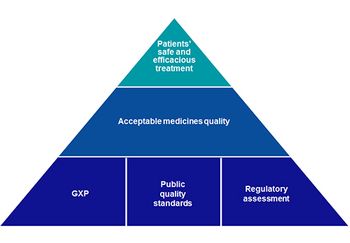
MHRA has launched a consultation to gain a greater understanding of the potential application of AQbD in pharmacopoeial standards and the future of medicines standards.

MHRA has issued a drug alert for the Emerade adrenaline auto-injector device, as a result of the device’s potential to fail in delivery of the dose due to blockage of the needle.

The US Pharmacopeial Convention is partnering with the American Association of Colleges of Pharmacy to provide students with free access to quality standards.

Altaire is voluntarily recalling ophthalmic products because of concerns regarding quality assurance controls in the manufacturing facility.

FDA sent a warning letter to Ecometics, Inc. after the agency found CGMP violations at the company’s Norwalk facility.

The draft guidance describes how content should be organized in electronic submissions for all submission types under section 745A(a) of the FD&C Act.

Slovakia becomes the final European Union country to be recognized by FDA, and the mutual recognition agreement for inspections of manufacturing sites between the US and the EU is now fully implemented.

The new guidance offers information for new drug application and biologics license application sponsors regarding population pharmacokinetic analysis.

The FDA guidance defines changes to approved risk evaluation and mitigation strategies and clarifies submission guidelines.

The draft guidance provides industry with a guide for using the database to assist in the development of drug products.

FDA released draft guidance on using the USP pending monograph process in the drug application process.

The guidance document provides recommendations for developing content for Instructions-for-Use documents for human prescription drugs, biological products, and drug-device or biologic-device combination products.

FDA sent warning letters detailing violations of current good manufacturing practices to three companies that repack APIs.