
FDA clarifies GDUFA requirements in regards to abbreviated new drug applications and prior approval supplements.

FDA clarifies GDUFA requirements in regards to abbreviated new drug applications and prior approval supplements.
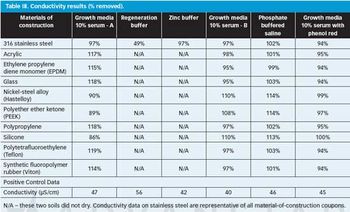
The authors look at the cleanability of pharmaceutical soils from a variety of materials of construction to determine the relative ease of cleaning and explore potential grouping strategies as part of a comprehensive cleaning validation program.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses how to ensure data integrity.
Nitrogen dioxide can sterilize and depyrogenate an aseptic fill area in a blow-fill-seal process.

Is there potential for growth in Brazil's phytotherapic drug market?

Stakeholders face challenges and benefits from a more secure pharmaceutical supply chain.

FDA releases guidance documents and rules on requirements for compounding human drug products.

European Medicines Agency clarifies advanced-therapy medicinal products classification.

Bristol-Myers Squibb issues a voluntary US recall of Coumadin (warfarin sodium) for injection due to presence of particulate matter.

GSK Biologicals receives warning about cGMP compliance issues at its Quebec facility.

FDA cites cGMP violations for API manufacturing at a facility in Tianjin, China.

FDA inspection found cGMP violations in the Bangalore, India API manufacturing facility.

ISPE Metrics Pilot Program is designed to demonstrate the feasibility and value of standard quality metrics.

FDA provides advice to supply-chain stakeholders on how to identify suspect drug products and how to notify the agency of those products.

EMA has published a new guidance template for the qualified person’s declaration concerning GMP compliance of API manufacture. The QP declaration template provides the basis for demonstrating compliance of the API manufacture with GMP requirements and ensures that the manufacturer has sufficient knowledge on the supply chain.

Draft guidance from FDA includes information essential for the completion of ANDA applications.

Despite GMP deficiencies, EMA reinstates GMP certificate for Ranbaxy's Toansa facility, citing no threat to public health.

EMA publishes revised guideline on the acceptability of names for drugs.

Regulators and industry organizations explain policies and standards to manufacturers and authorities in all regions.

Concern by environmentalists, regulators and manufacturers rises over the environmental impact of pharmaceuticals.

In legacy facilities and as buildings age, ensuring cGMP compliance can become complex. A review of a facility's gowning operations can bring insight into the current state of cGMP compliance. The author presents characteristics to look for and questions to ask.

An established drug-manufacturing base with quality and safety issues, and patent protection and price capping concerns complicate efforts to do business in India.

FDA finalizes guidance on expedited programs for new drug approvals for treatment of serious and life-threatening conditions.

Pfizer will submit a NDA with FDA for palbociclib, a treatment for locally advanced or metastatic breast cancer.

FDA issues complete response letter for Novartis' RLX030 for acute heart failure.