
High technology assessments are having an impact on biosimilars development in Europe.

High technology assessments are having an impact on biosimilars development in Europe.

ISPE and PDA take on the challenge of recommending quality metrics.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses how human error can be mitigated in pharmaceutical manufacturing.
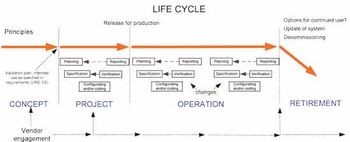
Non-compliance issues show that users find dealing with computer systems challenging.

Some recent high-profile cases of quality issues at Indian manufacturers have given reason to examine manufacturers more closely.

EMA's guidance focuses on the use of pharmacogenomics to improve drug safety monitoring.

India has a name when it comes to generic drug development. According to a recent research on patent applications carried out by Withers & Rogers, innovation by Indian pharmaceutical companies has increased over the past few years; however, the quality did not match the standard seen in Europe.

FDA adds Ranbaxy's Toansa, India facility to existing consent decree, prohibiting distribution of APIs from that location

Online portal accepts nominations for FDA advisory committee membership.

FDA releases guidance on developing drugs for the treatment of community-acquired bacterial pneumonia.

EMA has launched a new version of the EudraGMDP database, which includes the publication of statements of non-compliance with good manufacturing practice.

Brazil is the first Latin American country to emerge as a global biopharmaceutical collaborator.

Regulators work with manufacturers to improve systems for measuring product performance and manufacturing reliability.

Different approaches for qualification of personnel for visual inspection of residues on equipment surfaces are reviewed.

Kurt Lumden, Director, Client services at PAREXEL's Perceptive Informatics, discusses the management of investigational product temperature excursions.

With SMEs gaining wider recognition as the powerhouse of research and innovation in Europe, regulatory agencies are urging companies to engage with regulators early in the drug-development process.
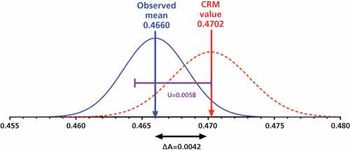
This article takes a statistical look at the calibration requirements for a UV spectrometer.

FDA and EMA launch initiative to share bioequivalence inspection information.

Agency issues precautionary recall due to manufacturing fault.

Agency issues precautionary recall due to manufacturing fault.

OGD is under pressure to improve review operations.

Teva Pharmaceutical Industries releases a financial outlook for 2014 based on two possible scenarios concerning its multiple-sclerosis drug Copaxone (glatiramer acetate).

Siegfried Schmitt, principal consultant at PAREXEL, discusses the state of drug manufacturing in India.

The European Union is strengthening its pioneering role in the regulation of biosimilars by further developing the basic rules for determining the levels of compatibility for this group of drugs. There are, however, some key issues that are not easy to resolve, as evident in a recent workshop on biosimilars organized by the European Medicines Agency (EMA).

PIC/S reviews deficiencies found during inspections of API manufacturing facilities, harmonizes GMP standards, and provides training for inspectors worldwide.