
Fewer field offices and inspectors will increase reliance on manufacturers to ensure product and process quality.


Fewer field offices and inspectors will increase reliance on manufacturers to ensure product and process quality.

Brussels, Belgium (Mar. 22)-The European Commission?s (EC) Directorate-General for Enterprise and Industry (Brussels, Belgium) is asking manufacturers, distributors, and users of human-pharmaceutical excipients to participate in an online questionnaire on the effect of various policy options. Responses will be used to prepare a directive on good manufacturing practices (GMPs) for certain excipients.

Forty years ago, investors and business speculators shuddered at the prospect of working with India's pharmaceutical industry. The industry was plagued by archaic patent laws and insufficient infrastructure, and only multinational companies (MNCs) were able to exploit its crude resources and monopolistic legal framework. Struggling in the shadows of these MNCs were the Indian pharmaceutical entrepreneurs and a handful of producers. Because more than 70% of the pharmaceutical market value was in the hands of MNCs, India's indigenous pharmaceutical industry was floundering. Its amount of exports was negligible, and the domestic market outlook was bleak because of onerous government regulation.

The enactment of the Indian Patents Act of 1970, implemented in 1972, provided an open platform to the Indian pharmaceutical industry to adopt process patents to manufacture active pharmaceutical ingredients (APIs) and formulations without fear of infringement of product patents. This resulted in a phenomenal growth in the number of pharmaceutical manufacturing units, from 2257 in 1970, to 5156 in 1980, 16,000 in 1990, and more than 23,000 in 2005. This was accompanied by a steep increase in investment from Rs. 2.25 billion (approx. $250 million US) in 1973, to Rs. 45 billion (approx. $1 billion US) in 2002–03. The prices of the most advanced drugs dropped significantly in India, leading the Indian pharma sector to become more competitive while remaining extremely cost effective in the global market.

Mishaps in packaging labels serve as a reminder: the recall is in the details.

FDA is buried in postapproval manufacturing submissions and seeks to reduce the scope of changes that require agency scrutiny.
This article considers the distinction among the terms qualification, validation, and verification in the context of pharmacopeial usage.A recommendation for a standardized usage of the terms validation and verification is provided,and general requirements for validation and verification activities are given.The article also emphasizes the importance of knowing when validation or verification is necessary relative to the use of a method to satisfy pharmacopeial article requirements (for which a monograph exists in the pharmacopeia) or for nonpharmacopeial use.

Indian pharmaceutical machine manufacturers (IPMMs) are exceptional among their foreign counterparts. Historically similar to the Chinese with regard to copycat practices, patent infringements, and substandard quality, the IPMMs have made great strides in innovation and collaboration to break free from the shackles of this paradigm.

Missed or late calibration dates can accumulate, and even if the equipment is labelled appropriately, it can suggest poor management of resources and priorities.

Just because the wheels are turning doesn't mean they're going forward.

FDA's new safety program, Critical Path Initiative, and user-fee proposal seek to reinvigorate pharmaceutical R&D.

"Quality by design" (QbD) and "quality risk management" at long last seem to be moving from the buzzword stage to becoming important influences on drug development and manufacturing. A series of quality standards issued by the International Conference on Harmonization (ICH) is encouraging the adoption of common quality-based drug manufacturing approaches designed to reach the "desired state" of drug manufacturing (i.e., more efficient, agile, flexible operations that can reliably produce high-quality drug products with less regulatory oversight). These developments reflect increased pressure to make pharmaceutical manufacturing more efficient and less wasteful and to encourage regulators in all regions to focus on the most critical issues affecting product quality and patient safety.

It's what's on the outside that counts, too.

An adulteration limit of 100 ?g/25cm? (4 ?g/cm?) was proposed for pilot-plant facilities. The dynamic changes in equipment, formulation, and residue determination made implementation of a constantly changing, calculated adulteration limit impractical. A single adulteration limit was simpler to communicate and document, making compliance achievable. The limit would be used only after it was determined to be lower than a health-based evaluation and a visual-cleanliness assessment.

Democrats are back on top in Congress and are mapping a broad agenda for change. Prescription drug pricing, medical product safety, and access to needed treatments are high on the priority list. Manufacturers will be in the hot seat answering questions about patent practices, high-risk products, and why drugs cost less in other countries than in the United States. The real challenge, however, will be to gain approval of a bill to reauthorize the Prescription Drug User Fee Act (PDUFA) before the program expires on Sept. 30, 2007. Such legislation also would renew user fees for medical devices and continue the pediatric drug exclusivity program.

During the past decade, the pharmaceutical industry has increased its use of information technology (IT) in research and development, production, and commercialization of pharmaceutical products. IT systems must be operated and maintained within a compliance-oriented framework to minimize risks; maximize safety and security, integrity, accuracy, reliability of information; and maintain product quality, For IT vendors and service providers, meeting requirements for qualification and validation calls for substantial investments in terms of creating capability, expertise, and resources. The author discusses the implications, challenges, and solutions in managing IT infrastructure qualification and validation in an FDA-regulated environment, particularly at vendor sites.

The production staff was sure the lab couldn't test their way out of a paper bag.

Pharmaceuticals current good manufacturing practice (CGMP) violations accounted for just 36 of the 441 Warning Letters issued by the US Food and Drug Administration in 2006.

Brand-name manufacturers seek to protect their products.

We really knew what we were doing-until we opened the column for a routine repacking.

EFPIA's 'Mock P.2' document aims to show how the role of 'quality risk management' and process analytical technology as an enabler for quality by design can be presented in a common technical document format. This article summarizes the main features of this document, and explains the key concepts and principles used.
The correlation between swab assay results and visible-residue limits (VRLs) for cleaning validation was examined. Previously completed validation studies were reviewed to compare swab results with recently determined VRLs. A current cleaning validation study evaluated both swab testing and VRL. Unexpected swab results led to an investigation, which showed the value of establishing the VRL in conjunction with swab recoveries for cleaning validation programs.

Here's to all the difficult, out-of-touch, and irresponsible coworkers that make our workplace interesting.

FDA must monitor a growing number of facilities and complex drugs despite "resource challenges."
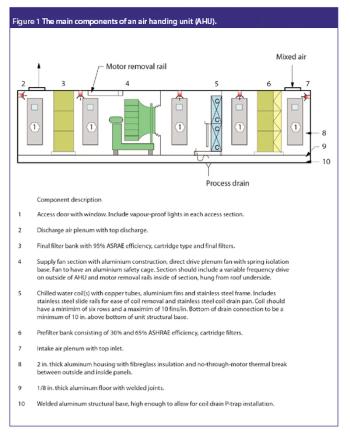
Clean rooms are critical areas in bio/pharma facilities, and it is essential that users are responsible for their care and upkeep, and familiarize themselves with the relevant regulations.