
India and China Position for Growth in APIs

India and China Position for Growth in APIs

Adding a cleaning step to the field-testing protocol, and combining it with the data generated to register sanitizing and disinfectant agents under FIFRA and the CEN TC 216 work program, produces a sanitation-and-disinfection validation methodology that is cost-effective, simple, and time-saving.

Madness in March: lost ingredients, missed lot numbers, and a million-dollar photo.

A new FDA policy emphasizes quality control over GMP compliance in producing clinical trial supplies.
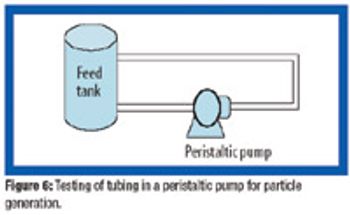
Because of the growing popularity of single-use materials, the identification, characterization, and qualification of new materials used for disposable processes have become increasingly important for both regulatory and production purposes. This article describes one approach to identifying and validating the materials used in a disposable filling process.
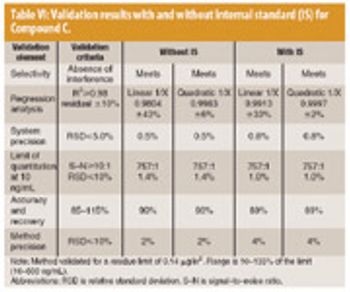
LC–MS–MS has the potential to become a routine technique for determining drug residues in support of cleaning verification, especially early in drug development.

"It was the late 1970s," reports our GMP-Agent-in-Place, "and we used a primitive desktop computer with built-in teletype for our quality control work.

FDA is proposing revisions to the drug user fee program while it weighs changes for drug safety initiatives and expanded vaccine production.

Warning: Brazilian Diet Pills Found to Contain Active Drug Ingredients

FDA Issues Dispute–Resolution Guidance

Schering-Plough Meets FDA Deadline for CGMP Improvements

Manufacturers, FDA, and research organizations are collaborating on efforts to spur innovation and streamline drug production.
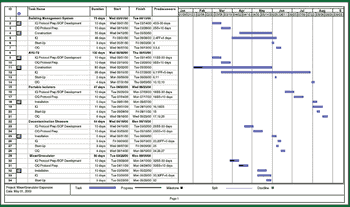
In the second half of this two-part series, the author suggests that to qualify and validate a pharmaceutical manufacturing facility, one must coordinate protocol and SOP development, scheduling and implementation, turnover package preparation, and the management and resolution of deviations and discrepancies. In combination with the programs described in Part I, these activities will help deliver projects on schedule, at estimated cost, and with quality assured.

A particular dermatological product was packaged in a metal tube, which is filled from the bottom.

To qualify and validate a pharmaceutical manufacturing facility, one must carefully review the facility design for compliance with good manufacturing practices and manage project scope definition, labor and cost estimating, and master-plan development.These activities, properly implemented, help deliver a validated facility on schedule, at the estimated cost, and with expected quality.

A new oral dosage product was designed as "encapsulated tablets." In production, the drug product was pressed into tablets, which were then fed into a revolving capsule-filling table.

CDER is reorganizing staffs and developing new policies to encourage industry adoption of quality production systems.

In applying a visually clean standard, any residue related to the cleaning process that is visible on the surface should constitute a failure.

New FDA Guidance on Electronic Common Technical Document Submissions

"It was just after FDA had initiated the Pre-Approval Inspection process," reports our GMP Agent-in-Place.

New leadership must address political and scientific issues as the Medicare drug benefit alters manufacturer incentives.
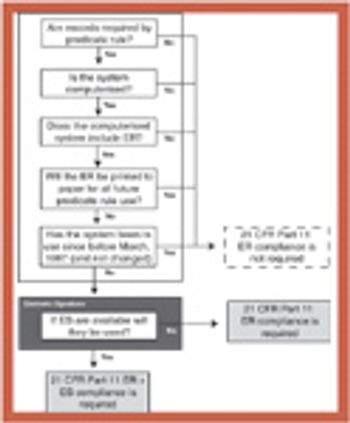
Coordinating validation efforts throughout an organization requires an accurate and timely overview and a validation master plan (VMP).
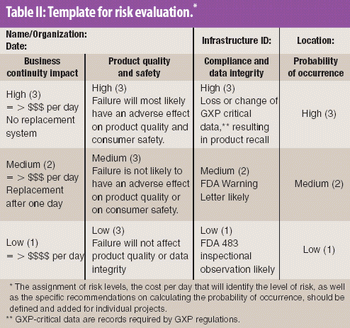
Risk analysis and evaluation of software and computer systems is a good tool to optimize validation costs by focusing on systems with high impact on both the business and compliance.
Performing studies to mathematically 'correlate' swab and rinse sampling values does not add any value. What's more, do not expect them to mathematically correlate.

von Eschenbach Drops NCI Hat…for Now