
A recent analysis found that 9000 marketed drugs are not in FDA's National Drug Code Directory.

A recent analysis found that 9000 marketed drugs are not in FDA's National Drug Code Directory.

Getting "swamped at work" takes on an entirely new meaning for these GMP Agents.

Developed in the 1950s as a means to survive and compete against the giants of the automotive sector, lean manufacturing helped Toyota evolve from a small-volume producer (with little capital) to become a high-volume manufacturer in a process-rich environment. Toyota achieved this by using developments such as total production maintenance (TPM), just-in-time (JIT), Kanban, value stream mapping and Kaizen events.1 A summary of some of the lean terminology is shown in Table 1.

Washington, DC (Sept. 1) - In a Federal Register announcement the US Food and Drug Administration laid out its guidance agenda for the coming months.

As the pace of product development accelerates, the approach to dissolution-method development must advance beyond a manual method and an assay. A natural progression of the method-development process must include the transfer of the manual method onto automated instrumentation.

Before formal cleaning validation programs were instituted, visual inspection was the primary means of determining equipment cleanliness. The use of visual inspection is still typically a component of a cleaning validation program and for routine inspections of cleaning effectiveness, but the use of visual inspection as a sole criterion for equipment cleanliness has not been successfully implemented as a valid approach for cleaning validation.

Apparently, the inspector would sneak off to visit his relatives on FDA time, instead of visiting us.

Partnerships launched 63 research projects that may translate into nine or 10 new drugs by 2010.

Implementing an electronic system to track out-of-specification results could help ensure compliance with current good manufacturing practices, but the system must be 21 CFR Part 11 compliant and easy to install, maintain, and use.

A contract manufacturing organization must clearly identify the goals of a project, establish a plan, and then effectively execute accordingly.

The US Food and Drug Administration has published a draft guidance, Q4B Regulatory Acceptance of Analytical Procedures and/or Acceptance Criteria (RAAPAC).

Thanks to you, we have this month's column. Keep those cards, letters, and e-mails coming.

The new drug user-fee program will define how FDA does business over its next 100 years.

The US Food and Drug Administration today posted its final "Guidance for Industry: Providing Regulatory Submissions to the Center for Biologics Evaluation and Research (CBER) in Electronic Format: Lot Release Protocols."
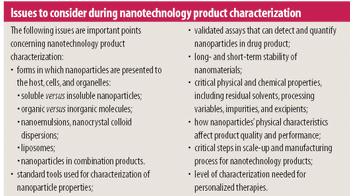
FDA is conducting laboratory research to understand better the ability of preclinical screening tests to identify potential risks and toxicities of nanotechnology-based drugs.

My audit host clearly knew and lied about the planned changes.

On June 15, 2006, the US Food and Drug Evaluation?s Center for Drug Evaluation and Research (Rockville, MD) issued a 7-page warning letter to Ranbaxy Laboratories (Himachal Pradesh, India) for violations to US current good manufacturing practices.

Noting several violations in current good manufacturing practice regulations, the US Food and Drug Administration has issued a Warning Letter to Wyeth Pharmaceuticals?s (Collegville, PA) manufacturing facility in Puerto Rico.

The US Food and Drug Administration (Rockville, MD) is withdrawing seven guidance documents "because some of the principles in these guidances are inconsistent with the agency's initiative, Pharmaceutical Current Good Manufacturing Practices (CGMPs) for the 21st Century (CGMP Initiative) .

I always suspected that our purchasing manager had agreed to this just to save money . . .
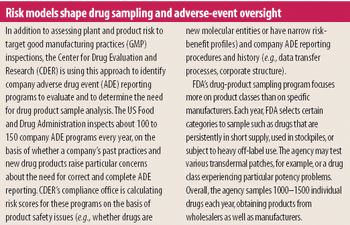
ORA leadership looks to staff redeployment and risk management to ensure product quality despite diminishing resources.

The CDER and CBER have released a new "Guidance for Industry: Q8 Pharmaceutical Development," outlining what drug manufacturers should include in the Pharmaceutical Development section of International Council on Harmonization (ICH) Common Technical Document (CTD) submissions.

Research and Markets (Dublin, Ireland) has released ?Annual Investment Analysis Report of the Chinese Pharmaceutical Logistics Industry, 2005-2006.? The report provides analysis of the pharmaceutical logistics characteristics, conditions, pharmaceutical market and pharmaceutical retail dynamics.

In a May 2 Federal Register notice (1), the US Food and Drug Administration withdrew its Jan. 17 direct final rule, "Current Good Manufacturing Practice Regulation and Investigational New Drugs" (2), which would have exempted manufacturing of drugs for Phase I clinical trials from most provisions of 21 CFR 211.

Sigma-Aldrich Corporation (St. Louis, MO) has acquired Iropharm, Honeywell International's custom chemical synthesis business in Arklow, Ireland. Terms of the cash purchase were not disclosed.