
New FDA act reshapes drug development and marketing to restore public trust in pharmaceutical regulation.

New FDA act reshapes drug development and marketing to restore public trust in pharmaceutical regulation.

The senior director of Oracle's Life Sciences Business Unit tackles some of the technical issues regarding regulatory standardization, software integration, and the trend toward visualization, among other things.

The US Food and Drug Administration sent Med-South Pharmacy a Warning Letter for producing large volumes of betamethasone acetate?betamethasone sodium phosphate without following current good manufacturing practices (CGMPs).

The US Food and Drug Adminstration?s Center for Biologics Evaluation and Research has issued a Warning Letter to Genzyme Corporation.

The US Food and Drug Administration launched a new program to increase the number and variety of generic drugs available to the public, beginning in FY2008.

As China emerges as a significant supplier of pharmaceutical ingredients, it must assure other countries of the safety of its excipients.

Our files pull up lost contracts, poor competitive tactics, and an over-ambitious employee.

Expanded access to drugs for seniors has increased demand and focused attention on costs.
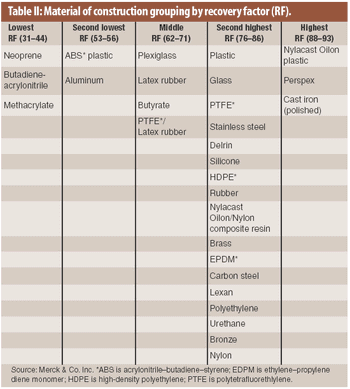
The material of construction is a factor in the recovery of residue in cleaning validation. An analysis of existing recovery data showed that recovery factors for drug products on various materials of construction may be categorized into several groupings.

The US Food and Drug Administration issued a final guidance, Manufacturing Biological Intermediates and Biological Drug Substances Using Spore-Forming Microorganisms, which provides recommendations that allow for greater flexibility when manufacturing biological products with spore-formers.

User-fee legislation will require more testing and data disclosure to prevent unsafe drug use.

Poor processing and misguided projections lead to trashed product.

The recently published Orange Guide 2007 contains significant changes to the GMP requirement placed on pharmaceutical manufacturers, but there have been additional changes to good distribution practice that should not be overlooked.

The authors discuss how companies facing ever-evolving regulatory requirements can address and assess compliance risk in their operating practices.

FDA's long-awaited GMPs for supplements appear as food and drug safety concerns override lingering opposition.

Production sometimes follows the law of supply and reprimand.
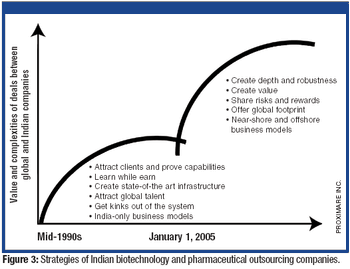
The tightening of intellectual property rights in India under GATT/TRIPS was a crucial inflection point for pharmaceutical outsourcing in India.

A new guidance issued by the US Food and Drug Administration earlier this month advises companies on how to treat polymorphic drug compounds?those that exhibit multiple structural forms?in filing abbreviated new drug applications.
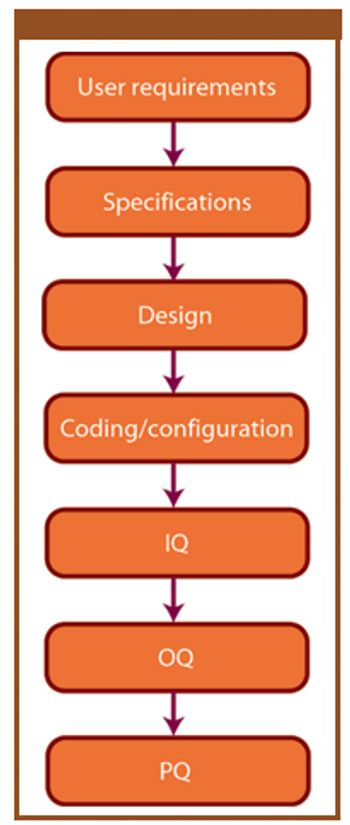
This article provides a historical review of computer validation in the pharmaceutical industry within the last three decades, evolving from the early years' initial concept and approach to today's current practices. Also included is how the regulations and industry have progressed in addressing the topic of computer validation.

This article looks at the current good manufacturing practice regulations from a statistical perspective while addressing their requirements and implications and inviting the industry to assess its past performance in meeting the regulations.

The good ol' days weren't always good.

Rockville, MD (May 31)-The US Food and Drug Administration issued a draft guidance on how to design bioequivalence (BE) studies for specific drug products to support abbreviated new drug applications (ANDAs).

Rockville, MD (May 31)-The US Food and Drug Administration finalized guidances for seasonal and pandemic influenza vaccines, outlining the regulatory pathways for developing and approving these products.

Look ahead to keep from falling behind.

Product characterization and production challenges are key issues in developing a pathway for biosimilar therapies.