
With rising drug deveopment costs and burdensome clinical trials, Indian-based firms are transferring their research departments to other entities in hopes of saving cash, mitigating risk, and ultimately, buying back the rewards.

With rising drug deveopment costs and burdensome clinical trials, Indian-based firms are transferring their research departments to other entities in hopes of saving cash, mitigating risk, and ultimately, buying back the rewards.

With counterfeiting on the rise and Europeans worried their backyard is becoming a base for such illegal activity, legislators have proposed a series of solutions that span the continent and abroad.

Regulators face demands to improve postmarket surveillance and meet review deadlines.

WFI system deficiencies and damp tax records cause problems for two plants

Problems associated with contamination of heparin products continue after worldwide recalls in March in the United States, Italy, France, and Denmark.

The US Food and Drug Administration issued a final guidance last week regarding investigational new drug applications for human gene therapy.

The US Food and Drug Administration withdrew a direct final rule that changed current good manufacturing practice (CGMP) regulations for finished pharmaceuticals. The agency withdrew the rule because it received significant adverse comments from industry.

Essential components of a good inspection: good ingredients, proper inserts, and ... deer?

Regulatory agencies expect companies to establish and monitor clean equipment- and dirty equipment-hold times for manufacturing equipment as part of their cleaning validation program.

Undertaking process validation involves a major commitment in terms of personnel, resources, time, and money. Performing prerequisite verifications can reduce the risk of making costly mistakes. This Part 1 article explains the value of performing prerequisite verifications and presents case-study examples and real-world solutions to avoid costly process validation failures.

Heparin contamination casts a shadow on regulatory oversight of product quality.

Quality-by-design submissions may reduce supplements and improve change management.

A. Nair discusses patent disputes in India.

If not properly monitored, filters and plastic bags can keep back more than they should.

The US Food and Drug Administration released a final guidance on protocol for testing sterile products.

The US Food and Drug Administration posted on its website a Jan. 24 Warning Letter to Novartis Vaccines and Diagnostics.
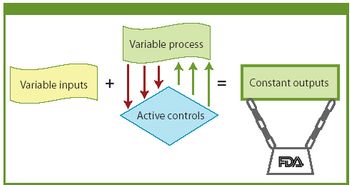
FDA is modernizing and streamlining current good manufacturing practices. The author examines FDA's evolving approach to quality systems and how a manufacturer can implement a quality system framework.

The rise in overseas manufacturing undermines FDA oversight of drug quality.

If at first the product fails, then inspect, inspect again

A wave of pharmaceutical expansions is expected in Europe this year, surprisingly by Indian companies.

The US Food and Drug Administration approved 69 new drug applications last year, the second lowest number of approvals in the past decade, according to the Jan. 15 edition of Drug Industry Daily.

The US Food and Drug Administration released a draft plan for modifying its information technology infrastructure. The plan follows the renewal of the Prescription Drug User Fee Act (PDUFA IV).

The $6.3 billion Indian pharmaceutical industry is at a crossroad. Aiming to be the international home for quality drugs, which could in itself propel India's market to $20 billion by 2015 according to recent estimates, the generic hothouse is clearly moving beyond its earlier low-cost mindset.

There is a need for current cleaning validation methods to be used for topical formulations. The authors highlight the issues and challenges encountered.

Process efficiency is measured not only by what is kept, but also by what is thrown away.