Although the European Union's approval rates of biosimilars for market is increasing slower than expected, its approach may provide an example for other foreign markets.

Although the European Union's approval rates of biosimilars for market is increasing slower than expected, its approach may provide an example for other foreign markets.

FDA has issued a Final Rule titled "Amendments to the Current Good Manufacturing Practice Regulations for Finished Pharmaceuticals."

Differential pricing is making more than just an appearance in Asia's pharma market these days.

FDA is taking several measures to ensure that imported drugs meet manufacturing standards.

Operators are hit hard by breakage problems and strike out on FDA inspections.

The authors describe the critical aspects of an ideal fermentation services provider.

FDA has finalized a regulation regarding changes to an approved new drug application (NDA), biologics license application (BLA), or medical device premarket approval applicatin (PMA).

The US Pharmacopeial Conventional added to its international sites with the official opening of its facility in Sao Paulo, Brazil.

The concept of the the triple bottom line incorporates a company's financial, social, and environmental performance. As a result, an increasing number of companies are investigating how sustainable, green technologies and practices can help them stay competitive in a challenging regulated market.

The US Food and Drug Administration issued a new draft guidance for public comment on sterile manufacturing.

Actavis Totowa LLC, the US subsidiary of the generic drug manufacturer Actavis Group, is announcing a voluntary recall to the retail level of all drug products manufactured at its Little Falls, New Jersey, facility. This is a precautionary, voluntary action by Actavis following an inspection conducted by the Food and Drug Administration earlier this year.

The US Food and Drug Administration has distributed a draft of Guidance for Industry: Residual Solvents in Drug Products Marketed in the United States for public comment.

Agents report unusual chemistry, abnormal data analysis, and unconventional work practices.

FDA is modernizing adverse-event reporting as part of a revolution in drug-safety assessment.

Bristol-Myers Squibb (BMS) agreed to reduce the output of ozone-depleting refrigerants at several industrial facilities around the country to resolve violations of the Clean Air Act. The company's modifications will cost approximately $3.65 million.

The US Food and Drug Administration published a Draft Guidance for Industry on Providing Regulatory Submissions in Electronic Format in the Federal Register on July 11.

Pfizer has agreed to pay $975,000 in civil penalties to resolve alleged violations of the Clean Air Act at its former manufacturing plant in Groton, Connecticut, according to a release by the US Department of Justice.

The European Pharmacopeia Commission has published the General Information chapter "Potentially Genotoxic Impurities and European Pharmacopoeia Monographs on Substances for Human Use" in the July 2008 edition of PharmEuropa.
A growing preference for public-private partnerships is spurring innovation and technology among Dutch pharmaceutical companies.

They may have seemed harmless at first, but these ideas took a big bite.

New scientific discoveries promise to expand treatments for rare and neglected diseases.
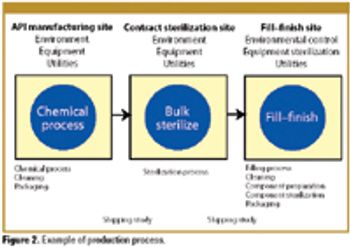
Patient safety must be the primary concern of any validation effort. The author explains how a risk-based approach to validation and compliance follows naturally from this premise.

With most deadlines missed for reviewing each abbreviated new drug application (ANDA) it receives, the US Food and Drug Administration is likely to take advice from the US Department of Health and Human Services (HHS) on how to speed up its process.

Effective July 1, 2008, the Office of Generic Drugs (OGD) will require abbreviated new drug applications (ANDAs) to include data that demonstrate the manufacturer's control of residual solvents.

The steering committee of the International Conference on Harmonization and its expert working groups adopted Quality Guideline Q10 "Quality Systems" last week at a meeting in Portland, Oregon.