
The app, CURE ID, is designed to allow the clinical community to report their experiences treating infectious diseases with novel uses of existing FDA-approved drugs.

The app, CURE ID, is designed to allow the clinical community to report their experiences treating infectious diseases with novel uses of existing FDA-approved drugs.

The agency has approved three applications for generic versions of Gilenya (fingolimod), Novartis’ blockbuster multiple sclerosis drug.
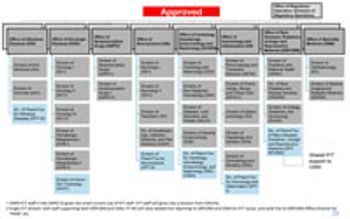
FDA’s Office of New Drugs restricting aims to improve scientific exchange and information sharing among review professionals.

A NASEM report stresses the importance of information sharing by biopharma companies and cooperation among regulatory authorities.

Regulators are facing huge challenges on how to deal with the digitalization transformation occurring in the healthcare and pharmaceutical sectors.

Problems in assuring reliable drug quality and supply dampens progress in bringing lifesaving therapies to market.

Investigating deviations of combination products needs to accommodate both the drug and device components, says Susan J. Schniepp, executive vice-president of post-approval pharma and distinguished fellow, Regulatory Compliance Associates.

The agency sent warning letters to 15 companies for illegally selling cannabidiol products.

The agency sent a warning letter to Torrent Pharma after an inspection found violations of current good manufacturing practices that included a failure to thoroughly investigate batch failures.

The guidance provides recommendations for the development and quality information that should be included in NDA and ANDA applications.

The approval of Givlaari (givosiran) is the first for treatment of acute hepatic porphyria, which results in the buildup of toxic porphyrin molecules during the production of heme.

The agency’s approval of Abrilada (adalimumab-afzb), a biosimilar to Humira, brings the total of approved biosimilars to 25.

The drug will be used to reduce the frequency of vaso-occlusive crises (VOCs) in adults and pediatric patients aged 16 years and older with sickle cell disease.

FDA issued a warning letter to the company for obtaining over-the-counter drugs made by foreign manufacturers that violated federal law.

Roche’s ADC, Kadcyla, has been recommended for approval in the EU for for the adjuvant treatment of people with HER2-positive early breast cancer with residual invasive disease after neoadjuvant treatment.

The recent approval by FDA of Vumerity (diroximel fumarate), a new drug for treating multiple sclerosis, has a triggered a $150-million milestone payment from Biogen to Alkermes.

NICE has issued a positive recommendation for GW Pharmaceuticals’ Epidyolex (cannabidiol) oral solution for the treatment of seizures in patients with a rare form of childhood-onset epilepsy.

Developers of biosimilars have become dismayed with difficulties in gaining acceptance and reimbursement from the US healthcare system.

In batch production, efficient exception management means reducing the time required to identify, review, and resolve process exceptions. Incorporating review by exception functionality within manufacturing execution system (MES) software can streamline biopharmaceutical product release.

Oncologist Stephen Hahn has been nominated for the top post, following the appointment of Brett Giroir as acting commissioner.

In a statement, Janet Woodcock MD, director of FDA’s Center for Drug Evaluation and Research, highlighted the agency’s efforts to enhance the efficiency of postmarket drug safety surveillance.

An effective quality control unit is independent from manufacturing and ensures current standards are followed.

FDA readies more efficient oversight processes while advancing collaboration with Europe.

It is good industry practice to clarify the precise remit for each of the reviewers of a controlled document, says Siegfried Schmitt, PhD, vice-president, technical, Parexel Consulting.

After years of discussions around pricing, UK governmental bodies and Vertex have finally reached an agreement on cystic fibrosis treatments.