
A year’s worth of FDA warning letters suggest that API and finished drug manufacturers should strengthen their approach to continued process verification.

A year’s worth of FDA warning letters suggest that API and finished drug manufacturers should strengthen their approach to continued process verification.

The recommended drugs include two orphan medicines and three biosimilars.

Commissioner Gottlieb is reorganizing FDA in the hopes of streamlining policymaking.

Rising pressure to do more to control drug prices is prompting Trump administration officials to explore where more flexible import policies may help ensure access to safe, effective, and more affordable medicines.

The agency announced two research collaborations on bulk lists, updates to categories of bulk drug substances, and issued a warning about a bulk drug substance used in compounding.

The agency has issued a warning letter for cGMP violations at a drug manufacturing facility in Ahmedabad, India, that Baxter gained through its acquisition of Claris Injectables.

The agency has approved Tpoxx (tecovirimat), the first drug with an indication for the treatment of smallpox, to address the impact of a potential outbreak.

FDA and ICH seek comment on new exposure levels for cadmium in drug products.

Inspectors found quality issues at bB BioChem Laboratories Inc, a California-based manufacturer of over-the-counter drug products.

New FDA guidance suggests ways to supplement labeling, to ensure that patients select and use nonprescription drugs safely.

FDA Commissioner, Scott Gottlieb, announced formation of a new, drug shortages task force and efforts to advance long-term solutions to prevent shortages.

Both parties have agreed to recognize each other’s inspection of manufacturing sites for a broader range of medicines, including sterile products, APIs, biologics, and vaccines.

Teva Pharmaceuticals, Major Pharmaceuticals, and Solco Healthcare are voluntarily recalling their products containing valsartan because of the presence of N-nitrosodimethylamine.

The agency is releasing six new draft guidances to provide a regulatory framework for handling gene therapies.

Certain blister card packages that do not meet US child-resistant packaging requirements are being recalled because they pose a risk of harm if the tablets are swallowed by children.

The agency is reviewing products containing valsartan supplied by Zhejiang Huahai Pharmaceuticals, a company in Linhai, China.

The European Medicines Agency recommended Novartis’ Kymriah (tisagenlecleucel) and Kite’s Yescarta (axicabtagene ciloleucel), chimeric antigen receptor (CAR) T-cell therapies, for approval in the European Union.

The agency published guidance on how the Generic Drug User Fee Amendments Reauthorization of 2017 applies to amendments to ANDAs and PASs.
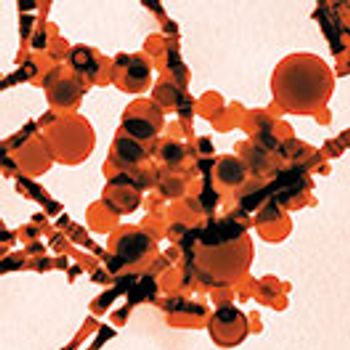
The delivery device and drug form should be considered when choosing a test method for identifying and measuring particulates in inhaled drug products.

The agency is asking firms to discuss internal quality metrics efforts as part of the approval process for new medical products.

Legislators look to widen access to medications for addiction treatment and overdose emergencies.

The European Directorate for the Quality of Medicines & Healthcare highlighted the organization’s achievements in 2017, including the first mAb monograph.

FDA seeks more efficient testing to spur development of less costly biotech therapies.

EMA, the leading driver behind GMP inspection collaboration schemes, will be cutting back its involvement because of the need to concentrate on its relocation to Amsterdam.

Knowing and addressing regulatory expectations early on can avoid unexpected delays later, says Siegfried Schmitt, principal consultant at PAREXEL.