
Shire has announced that the European Commission has granted marketing authorisation for Takhzyro (lanadelumab) subcutaneous injection.

Shire has announced that the European Commission has granted marketing authorisation for Takhzyro (lanadelumab) subcutaneous injection.

The proposal from the Centers for Medicare and Medicaid Services (CMS) proposes to give plans more flexibility to limit coverage of certain drugs.

A required time frame should not be the driving force behind root cause investigations, says Susan Schniepp, executive vice-president of Post-Approval Pharma and Distinguished Fellow, Regulatory Compliance Associates.
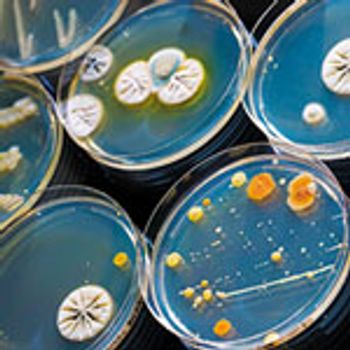
Microbial identity data can be critical for determining contamination sources.

Drawing on practical experience, the authors examine key questions and answers about various aspects relating to the enhanced approach for analytical procedure lifecycle management.

EMA’s relocation to Amsterdam and resulting staff losses could severely weaken the agency’s role as a leading medicines regulator.
GMP non-compliance can spill over and impact patient access to life-saving medications.

FDA Commissioner Scott Gottlieb has been promoting drug market competition in recent months that includes new guidance documents and targeted advisories to support R&D of complex drugs and combination products.

Owner of Immuno Biotech, David Noakes, has been sentenced to 15 months in prison over charges of manufacturing, selling, and supplying an unlicensed medicine, as well as money laundering.

Five additional European Union member states have been confirmed by the US Food and Drug Administration (FDA) as capable of performing good manufacturing practice inspections at a level equivalent to that of the United Sates

Despite ongoing efforts to address the problem, FDA now sees a rise in active shortages and in the duration of supply problems.

The agency has approved two new chemical entities, Daurismo (glasdegib) from Pfizer, and Vitrakvi (larotrectinib) from Loxo Oncology, for treating cancers.

FDA has issued a warning letter to Mylan citing GMP violations of finished pharmaceutical products manufactured at the company’s Morgantown, WV, facility.

A coalition group from the UK's health sector has called on both the UK and EU to ensure the protection of patients and public health when considering the future relationship of the regions post-Brexit.

GSK has filed for a sBLA to FDA for the expansion of the indication of Nucala to include paediatric patients between 6 and 11 years old.

During CPhI Worldwide in Madrid, Lynne Byers, executive director of NSF International detailed the facts about how Brexit can impact the pharmaceutical industry.

Pharma industry welcomes announcement of a draft withdrawal agreement, stating it is an important step towards securing a Brexit deal.

The positive opinions included the first oral-only tablet for the treatment of human African trypanosomiasis.

After a review of serious side effects, the agency decided to suspend marketing authorizations for quinolone and fluoroquinolone antibiotics and restrict use of remaining fluoroquinolone antibiotics.

Moving forward with gene therapy development requires a “quantum leap” in manufacturing capabilities.

The agency provided an update on its relocation plans and assured that core activities are continuing uninterrupted.

The new good pharmacovigilance practice chapter IV on specific considerations for the pediatric population offers a holistic view of pediatric pharmacovigilance and provides guidance on how to address the specific needs and challenges of safety monitoring of medicines used in children.

The agency sent a warning letter to the company for marketing an unapproved stem cell product and CGMP violations.

Improving the manufacturing of gene therapy vectors will be crucial to making advanced treatments accessible to more patients who need them, agreed panelists at the 2018 Galien Forum.

The agency is developing a new way to assess, record, and report data from surveillance and preapproval inspections of sterile drugs.