The COVID-19 pandemic is pushing more companies to adopt just-in-time practices, but success demands careful upfront risk assessment and planning.
Agnes Shanley is senior editor of BioPharm International.

The COVID-19 pandemic is pushing more companies to adopt just-in-time practices, but success demands careful upfront risk assessment and planning.

The COVID-19 pandemic has led to increased demand for direct-to-patient shipments, challenging cold-chain specialists to become more agile and to strengthen their global distribution networks.
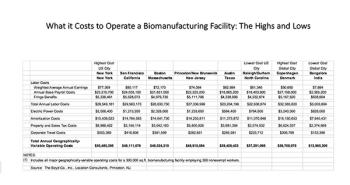
As politicians focus on drug cost reduction, biopharmaceutical companies in the US are moving to states with lower taxes, and relocating some facilities that had been offshore.

Now that the first genetically modified cell therapies are being manufactured, the industry must move beyond “whatever works” to meet growing demand.

The means to continually improving and enabling the use of artificial intelligence in manufacturing lies in the ability to make data accessible across functions and to contract partners.

Often, transferring an analytical method from R&D to quality control fails; success depends on determining where “the best” and “the most reliable” intersect.

A technology roadmap aims to drive and consolidate improvements in a process that has remained unchanged for more than 70 years.
Published: March 2nd 2021 | Updated:

Published: December 2nd 2017 | Updated:

Published: March 7th 2019 | Updated:

Published: July 2nd 2019 | Updated:

Published: August 1st 2019 | Updated:

Published: February 11th 2020 | Updated: