
The recent announcement that GlaxoSmithKline will pay a record $3 billion in civil and criminal penalties marks the largest settlement ever incurred by a drug company,
Amy Ritter was Scientific Editor, BioPharm International.

The recent announcement that GlaxoSmithKline will pay a record $3 billion in civil and criminal penalties marks the largest settlement ever incurred by a drug company,

Improvements in expression platforms and enhanced tools for selecting clones are among the advances of the past few decades.

A look back at key nanoformulation advances and what lies ahead for nanoparticle-based drug-delivery systems.

In a 5-4 ruling, the US Supreme Court upheld the provision of the Affordable Care Act requiring all adults to purchase health insurance or pay a penalty—the so-called individual mandate.

Roche announced that it will be closing its R&D site in Nutley, New Jersey, eliminating approximately 1000 positions.

Roche’s recent announcement that it will be closing its venerable Nutlley, NJ R&D site is the latest in a string of mergers and reorganizations that have resulted in the loss of thousands of life-sciences jobs from New Jersey,

Eli Lilly announced an increase in its manufacturing network in China through an expanded collaboration with Novast Laboratories, a generic and specialty pharmaceutical company based in Nantong, China.

Takeda Pharmaceuticals announced that it has completed the acquisition of URL Pharma for an upfront payment of $800 million.

The House Appropriations Committee released the fiscal year 2013 Agriculture Appropriations bill, which, sets proposed spending levels for FDA as well as for other programs
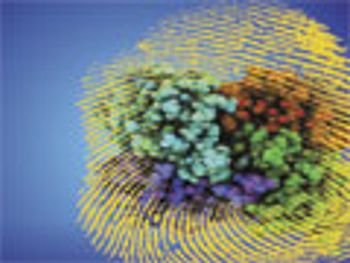
Extensive physicochemical characterization of the innovator product and the proposed biosimilar provides the foundation for demonstrating biosimilarity.

Osiris Therapeutics announced that it received marketing authorization from Health Canada to market its stem cell therapy, Prochymal, for the treatment of graft-versus-host disease in children.

Federal marshals seized an unapproved topical corticosteroid medication from California-based Crescendo Therapeutics.

With pharmaceutical R&D efforts in decline, public-private partnerships are emerging as a way to bolster early stage discovery efforts. In Europe, concern over a dwindling antibiotic pipeline led to the European Commission’s Action Plan Against the Rising Threats from Antimicrobial Resistance, launched in November last year (see here).

A theme shared by several of Wednesday’s sessions at the AAPS National Biotechnology Conference seems to be how the tools and techniques that have evolved for development of small molecules are spilling over into the design of large molecules.

AAPS Biotech is holding its annual meeting this year in San Diego, CA, the city with arguably the best weather in the world.

PDA held a successful workshop earlier this spring in Phoenix for over 100 attendees on key elements of the association’s forthcoming Technical Report on Single-use.

Senators Max Baucus (D-MT) and Chuck Grassley (R-IA) send letters to opioid manufacturers and pain groups asking them to disclose financial ties.

Abbott Laboratories will pay $1.6 billion to settle outstanding issues concerning past sales and marketing practices for its neurologic medication, Depakote.

The world of drug development is littered with early-phase failures: drugs that were shown to be safe in Phase I trials, but which failed to show efficacy later on.

At the request of FDA, the IOM prepared a report containing recommendations for how FDA might better monitor the safety of drugs after they have been approved.
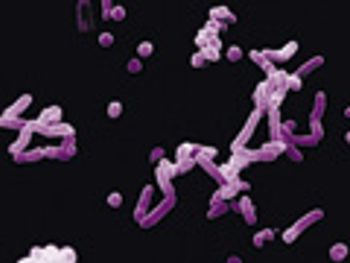
In this technical forum, experts describe different methods of rapid microbial testing and their applications.
Readers share their views on bioprocessing challenges, equipment use, and outsourcing trends in our annual bioprocessing equipment and processing survey.

Abbott Laboratories submitted a Citizen’s Petition to FDA, requesting that the agency not consider any applications for biosimilar versions of its monoclonal antibody therapeutic.

Apr. 16, 2012 marked the closing date for submitting comments on FDA’s draft guidance documents clarifying the approval process for biosimilars.

Here in sunny Arizona, PDA is holding its annual conference, a chance to present data and discuss all aspects of sterile manufacturing. The theme for the first day of sessions was personalized medicine.

FDA has released a final guidance describing cGMP for preparing media fills for validation of aseptic preparations for positron emission tomography drugs.

Ranbaxy Laboratories announced that beginning in March 2012, the first shipments of atorvastatin, the generic version of Pfizer's Lipitor, had been sent to US markets from its new Mohali manufacturing facility located in Punjab, India.

With around 80% of APIs manufactured outside the US, many in developing nations without strong regulatory oversight.

On Mar. 13, 2012, FDA issued a Warning Letter to Steven Victor, CEO of IntelliCell Biosciences for a violation of the Food, Drug, and Cosmetic Act, and for violations of cGMP and Good Tissue Practice in the manufacture of its adipose tissue-derived stem-cell product.

Personalized medicine is gaining traction as scientists gain greater understanding of disease processes.