
Teva Pharmaceuticals received a Warning Letter from FDA informing them that promotional materials as well as an associated webpage for Copaxone (glatiramer acetate injection) solution for subcutaneous injection were found to be false and misleading.
Amy Ritter was Scientific Editor, BioPharm International.

Teva Pharmaceuticals received a Warning Letter from FDA informing them that promotional materials as well as an associated webpage for Copaxone (glatiramer acetate injection) solution for subcutaneous injection were found to be false and misleading.

A critical drug that has experienced shortages is Genzyme’s, Fabrazyme (agalsidase beta), the only enzyme replacement therapy approved in the US for Fabry disease.

On Mar. 7, 2012, GE Healthcare announced an agreement to acquire Xcellerex, a supplier of manufacturing technologies for the biopharmaceutical industry, for an undisclosed amount.

This week, the Drug, Chemicals, and Associated Technologies Association (DCAT) is holding its annual week-long meeting in New York City.

Boehringer Ingelheim (BI) has announced the expansion of its biopharmaceutical manufacturing capabilities at its plants in Biberach, Germany and in Vienna, Austria. The expansion will include cell-culture and microbial-fermentation capacity, and support cell-line and process-development services for BI's contract manufacturing business.

A short time ago, I wrote about the Transforming the Regulatory Environment to Accelerate Access to Treatments Act, (TREAT).

FDA's treatment of whistleblowers lacks internal consistency.

Merck KGaA announced measures intended to reduce costs and increase efficiency to ensure the long-term success of its business model. The measures announced this week are part of a comprehensive transformation program that will be implemented in two phases.

Rare disease day is an annual awareness-raising event coordinated at the international level by EURORDIS, a non-governmental patient-driven alliance of patient organizations representing more than 502 rare diseases patient organizations in over 46 countries.

On Feb. 21, 2011, FDA posted a copy of a Warning Letter that was sent to the chairman of the executive board of Merck KGaA on Dec. 15, 2011, regarding cGMP violations identified at three of its European facilities: MS-Corsier-sur-Vevey, MS-Aubonne, and MS-Tiburtina.

Senator Kay R. Hagan (NC) introduced a piece of legislation called the Transforming the Regulatory Environment to Accelerate Access to Treatments (TREAT) Act.

FDA has released a draft guidance for API manufacturers in response to a 2008 incident in which heparin sourced from China was adulterated with oversulfated chondroitin sulfate, causing serious adverse reactions in patients.

FDA and Genentech, a member of the Roche Group, have issued warnings about counterfeit versions of the injectable cancer drug, Avastin, circulating in the US. According to the FDA safety alert, the counterfeit version of Avastin does not contain the medicine’s active ingredient, bevacizumab, which may have resulted in patients not receiving needed therapy.

GlaxoSmithKline released its fourth quarter and full year 2011 earnings report, which showed its return on R&D to be 12%-up from 11% in 2010, and closing in on the company's goal of a 14% return.

A report released on Feb. 8, 2012 from the California Healthcare Institute, BayBio and PwC shows that the shrinking economy, changes in investment strategies, and pressures on the pharmaceutical market have put the brakes on one of the US’s most robust biotechnology centers.

PhRMA announced that it will transition the management of its benefit–risk action team to the Centre for Innovation in Regulatory Science (CIRS). CIRS is a neutral, independent UK-based subsidiary company, forming part of the Intellectual Property and Science business of Thomson Reuters.

As part of Ranbaxy’s recently announced consent decree, the company is required to set up a program whereby whistleblowers can come forward with information related to potential violations of the Food, Drug, and Cosmetic Act. According to the decree, available through Pharmalot’s post, Ranbaxy must, within 75 days, establish a phone line and a system to receive and maintain submissions from individuals wishing to report suspected violations

Sanofi's subsidiary Genzyme has received FDA's approval for the production of Fabrazyme (agalsidase beta), used in the treatment of Fabry's disease, at its manufacturing facility in Framingham, Massachusetts. Shortages of Fabrazyme have been ongoing for the past two years.

When should there be restrictions on the dissemination of basic research results? This question has arisen in the context of papers from independent laboratories submitted to the journals Science and Nature.

On Jan. 11, 2012, AstraZeneca and IMS Health announced a three-year collaboration to use real-world healthcare data from Europe to inform AstraZeneca's discovery and clinical development programs.

The US Pharmacopeia announced a draft standard containing best practices for ensuring that drugs can be traced to their original manufacturer, are not counterfeited or adulterated, and can be transported to their intended destination without compromising quality.

A newly released report from Jones Lang LaSalle, a financial and professional services firm specializing in real estate services and investment management, looks at the world in terms of life-sciences investment, and finds some not-so-surprising trends over the past 10 years. The report ranked

The National Institutes of Health has established the National Center for Advancing Translational Sciences, a center dedicated to the translation of scientific discoveries into new drugs, diagnostics, and devices.

If a clinical trial is run, but the results are never published, what benefit is there to patients, physicians, or the public?

Creating a successful antibody-drug conjugate requires careful selection of the drug, antibody, and linker.

The Government Accountability Office issued a report recommending that FDA's ability to respond to drug shortages be strengthened. The report was released in conjunction with a Senate hearing before the Committee on Health, Education, Labor, and Pensions on Dec. 15, 2011, on the subject of drug shortages.

AstraZeneca entered into an agreement to acquire Guangdong BeiKang Pharmaceutical, a privately owned generic-drug manufacturing company based in China, for an undisclosed amount. Upon completion of the acquisition, AstraZeneca will be responsible for the manufacture and commercialization of these medicines.

On Dec. 6, 2011, FDA announced that a public meeting will be held on Dec. 16, 2011 to discuss recommendations for a user fee program for biosimilar biological products for fiscal years 2013–2017.

Readers react to the economic turmoil of the past year and look longingly forward to 2012.
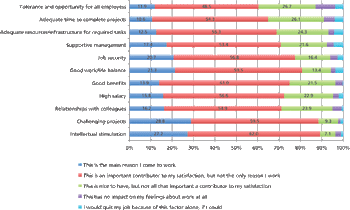
The results from our employment survey are in.