
Myth versus Reality: What does Q10 implementation really mean for my company?
Angie Drakulich was editorial director of Pharmaceutical Technology.

Myth versus Reality: What does Q10 implementation really mean for my company?
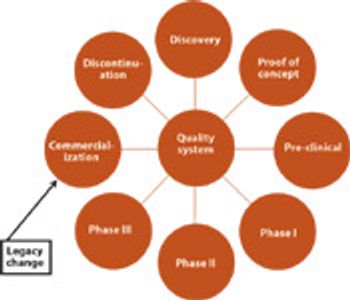
After five years in the making, the official pharmaceutical quality system is here. All three parties to ICH adopted a final version of Q10 and agreed to implement the guideline through their individual regulatory bodies.

The European Medicines Agency recommended strengthening the warnings for oral moxifloxacin medicines and concluded that these drugs should only be prescribed to treat acute bacterial sinusitis, acute exacerbation of chronic bronchitis, and community-acquired pneumonia when other antibiotics cannot be used or have failed.

The US Food and Drug Administration issued a final rule on July 18 that exempts early Phase I investigational drugs from certain good manufacturing practice regulations.

The US Food and Drug Administration published a Draft Guidance for Industry on Providing Regulatory Submissions in Electronic Format in the Federal Register on July 11.

The US Food and Drug Administration is seeking volunteers from the pharmaceutical industry to participate in a pilot program involving the submission of quality information for biotechnology products.
Staff reductions are not surprising with the state of the economy and falling pharmaceutical sales.

Forums, blogs, and more can spark innovation.
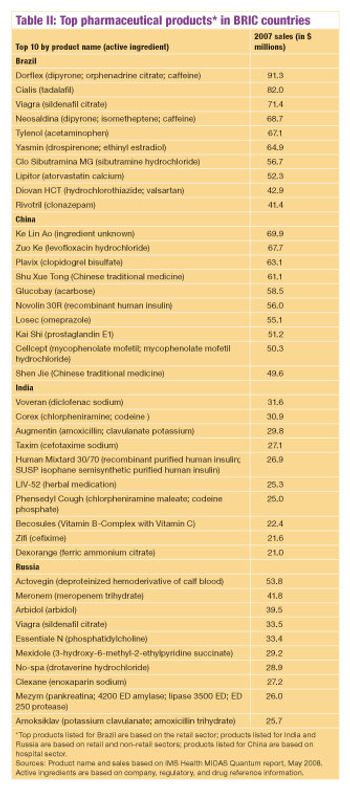
Recent reports discuss the future of pharma's emerging markets.

The US Food and Drug Administration will have a $2.1 billion budget next year if the fiscal year 2009 bill markup by the US House of Representatives' Agriculture Appropriations Subcommittee passes through Congress.

With most deadlines missed for reviewing each abbreviated new drug application (ANDA) it receives, the US Food and Drug Administration is likely to take advice from the US Department of Health and Human Services (HHS) on how to speed up its process.

The number of clinical-trial investigators overstepping their bounds has grown in the past few years. And, says Congress, the US Food and Drug Administration is taking too long to wrap up unresolved cases.

The steering committee of the International Conference on Harmonization and its expert working groups adopted Quality Guideline Q10 "Quality Systems" last week at a meeting in Portland, Oregon.

The US Food and Drug Administration issued a new guidance Monday on indexing structured product labeling. The Center for Biologics Evaluation and Research and the Center for Drug Evaluation and Research will begin indexing structured product labeling in the product labeling for human drug and biologic products.

Last week, 2704 delegates representing 190 nations met in Geneva for the 61st World Health Assembly to set a global course for tackling new as well as longstanding threats to public health worldwide. The assembly?s biggest breakthrough focused on adopting a resolution that encourages research and development (R&D) as well as access to medicines for people living in developing countries, according to a World Health Organization (WHO) release.

The World Health Organization's World Health Assembly is holding its 61st annual session this week to discuss a number of public health issues, many of which are tied directly to pharmaceutical manufacturing.

Once again, the US Food and Drug Administration is under fire for not doing its job. This time the issue is direct-to-consumer (DTC) advertising.

The European community and European Federation of Pharmaceutical Industries and Associations (EFPIA) held its first call for proposals for its five-year, €2 billion ($3.1 billion) initiative to boost biomedical innovation.

According to the latest figures from IMS Health, Inc., Japan's pharmaceutical market is expected to grow 1–2% this year compared to global industry growth expectations of 5–6%.

US Food and Drug Administration leaders issued another cry for help last week. Center for Drug Evaluation and Research Director Janet Woodcock testified before the Senate Health, Education, Labor and Pensions (HELP) committee at an April 24 hearing, "Restoring FDA's Ability to Keep America's Families Safe."

The US Food and Drug Administration issued a final guidance last week regarding investigational new drug applications for human gene therapy.

Senator Chuck Grassley (R-IA) sent a letter to Amgen (Thousand Oaks, CA) last week asking the company to account for the high rebates it has given to certain physician groups who bought Aranesp.

Filing risk and mitigation strategies is now a requirement for manufacturers of new drugs and biologics.

US Representatives Anna G. Eshoo (D-CA) and Joe Barton (R-TX) introduced legislation this month week to create a regulatory pathway for biosimilars, or follow-on biologics.

As part of ongoing efforts to improve the quality of imported drug products, the US Food and Drug Administration is setting up shop in China.

With another potential "made-in-China" crisis looming over the recall of heparin, critics and the media seem to be waiting in line to take another jab at the US Food and Drug Administration.

The US Food and Drug Administration released a final guidance on protocol for testing sterile products.

The US Food and Drug Administration issued a draft guidance for industry titled "Good Reprint Practices" regarding the distribution of medical or scientific journal articles and reference publications that involve unapproved uses of FDA-approved drugs and medical devices.

A follow-on biologics approval pathway may be on the way. US Health and Human Services Secretary Mike Leavitt accepted Sen. Charles Schumer's (D-NY) suggestion that the Food and Drug Administration and Congress work together to formulate legislation for such a pathway.

The US Food and Drug Administration is requesting a 5.7% increase in its budget between the current fiscal year, FY 2008, and FY 2009, for a total $2.4 billion budget.