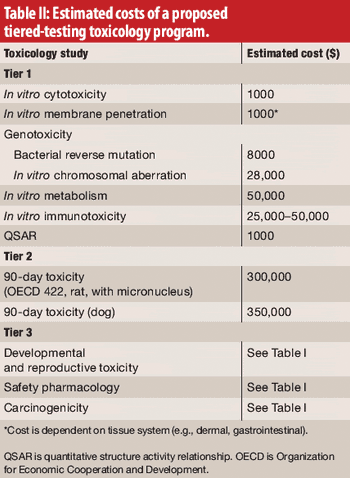
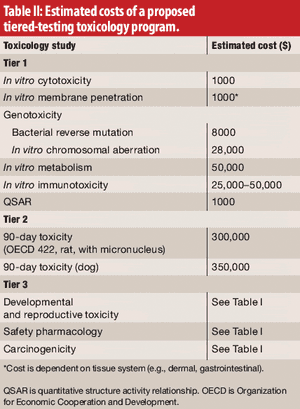
The authors, representing the International Pharmaceutical Excipients Council, propose a new evaluation procedure, including tiered toxicology testing for excipients.
Christopher C. DeMerlis is a regulatory affairs manager at Colorcon, a division of Berwind Pharmaceutical Services, Inc., 415 Moyer Blvd., West Point, PA 19486, tel. 215.661.2766, fax 215.661.2778.

The authors, representing the International Pharmaceutical Excipients Council, propose a new evaluation procedure, including tiered toxicology testing for excipients.

Natural gums and mucilage are biocompatible, cheap, readily available, and represent a potential source of excipients. The authors examine the functionality of mucilage extracted from the leaves of Hibiscus rosa-sinensis Linn as an excipient in a sustained-release tablet formulation.

The authors propose several models for independent evaluation of new excipients.

A global drug master file system may pave the way for faster qualifications of new excipients and the industry's acceptance of their use in drug therapies.
Published: November 2nd 2009 | Updated:

Published: January 2nd 2008 | Updated:

Published: June 1st 2002 | Updated:

Published: November 2nd 2003 | Updated: