
The authors present an update to the Wyeth/BASF experience with the IPEC Novel Excipient Safety Evaluation Procedure.
Ranga Velagaleti is the manager of laboratory and stability at BASF Corporation, 8800 Line Ave., Shreveport, LA 71106, tel. 318.861.8040, fax 318.861.8004, velagar1@basf-corp.com

The authors present an update to the Wyeth/BASF experience with the IPEC Novel Excipient Safety Evaluation Procedure.
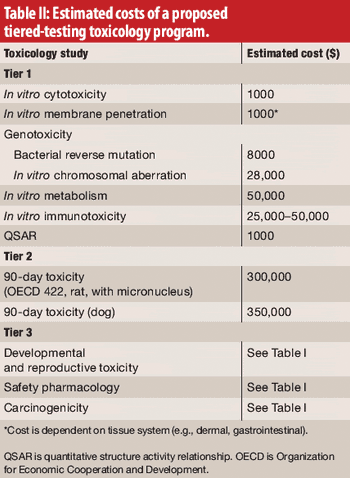
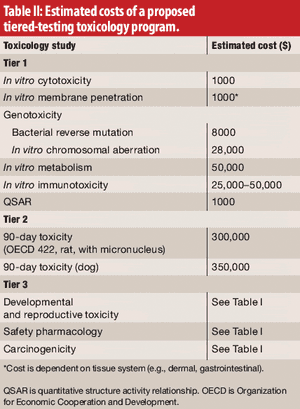
The authors, representing the International Pharmaceutical Excipients Council, propose a new evaluation procedure, including tiered toxicology testing for excipients.

The results of forced degradation studies indicate the need for alternatives to valerophenone as an internal standard calibration for quantifying ibuprofen in bulk drug and tablet assay samples.

The authors subjected ibuprofen bulk drug and tablet assay preparations to various stresses to evaluate the selectivity and specificity of the drug substance and product samples.

This study investigated the effectiveness of the direct extraction of tablets and shaking time on the disintegration of tablets, solubilization, and recovery of ibuprofen from tablets of various formulations, strengths, and spiked placebo.

Published: March 1st 2002 | Updated:

Published: November 2nd 2010 | Updated:
Published: November 2nd 2009 | Updated: