
Lee Cronin, founder and CEO of Chemify, discusses the combination of digital chemistry, robotics, and AI in the drug discovery space.
Feliza Mirasol is the science editor for Pharmaceutical Technology and Pharmaceutical Technology Europe.

Lee Cronin, founder and CEO of Chemify, discusses the combination of digital chemistry, robotics, and AI in the drug discovery space.

CGT Catapult and CATTI aim to standardize advanced therapy manufacturing with new aligned training standards.

CGT Catapult will implement Cellular Origins' robotic platform in its Stevenage, UK, site to establish automated CGT manufacturing.

Lilly’s investment increase is intended to boost manufacturing capacity at its Lebanon, Ind., site for APIs used in the production of tirzepatide and other pipeline drug candidates.

Johnson & Johnson will gain NM26, a bispecific antibody targeting atopic dermatitis, via the acquisition of a wholly owned subsidiary of Numab Therapeutics.

The partnership aims to provide end-to-end development and manufacturing for biopharmaceutical drug substance and drug product.

With the acquisition, Biogen gains felzartamab, HI-Bio's lead investigational mAb candidate being developed for the treatment of a range of immune-mediated diseases.

Bari Kowal, senior vice-president, head Development Operations & Portfolio Management, Regeneron, discussed the utility of AI and how it can benefit R&D work.

Ken Keller, president and CEO, Daiichi Sankyo, discusses the foundation of partnership and takes a look at the future of biotech partnerships at the US Pharma and Biotech Summit.

Phillip Gregory, PhD, senior vice-president and head of Regeneron Cell Medicines, Regeneron, discussed how engineering receptor architecture can be a tool to improve CAR-T cell sensitivity to tumor antigens.

Regeneron's Sven Moller-Tank, PhD, director of Viral Delivery Technologies, Regeneron Genetic Medicines, discusses the company's work in using bispecific antibodies for redirecting AAV target specificity.

Experiments conducted by the downstream technology team at Spark Therapeutics involving metal ion-containing additives showed improved capsid clearance in AAV production.

ProPharm and PBL have introduced a fully automated, enclosed cell factory manufacturing device.

The two companies will combine their cell therapy platforms to develop convertibleCAR programs targeting solid tumors.

Digital transformation is allowing for better handling, analysis, and protection of vast data collection.

A new draft guidance issued by FDA covers human- and animal-derived materials used in the manufacture of advanced therapy medicinal products.

In the new draft guidance, FDA provides recommendations on cell safety testing of human-sourced allogeneic cells for sponsor companies.

In this episode fo the Drug Solutions Podcast, etherna’s vice-president of Technology and Innovation, Stefaan De Koker, discusses the merits and challenges of using mRNA as the foundation for therapeutics in oncology as well as for vaccines.

SK pharmteco, a CDMO, will manufacture, test, and release Adstiladrin (nadofaragene firadenovec-vncg), a gene therapy from Ferring Pharmaceuticals for treating bladder cancer.

Mary Van Gaasbeck, technical services specialist, LS Equipment and Services, at STERIS Life Sciences, notes that automation is key when it comes to effective sterile powder transfer of parenteral drug products.

Andreas Frerix, product management director for Quattroflow at PSG Biotech, discussed the advantages and new challenges SUTs present for pumping systems.

Jay Rajagopalan, senior director—Engineering & Product Management for Malema at PSG Biotech, shared insights at on how current industry trends are shaping the development and advancement of sensor technologies.

Pharmaceutical Technology® spoke with Nicole Hunter, Watson-Marlow Fluid Technology Solutions’ head of Global WMArchitect, about the impact single-use technologies have on fluid-handling workflows in bioprocessing.

The improvement of ADC manufacturing now demands increased containment requirements and continually advancing analytical detection of molecules in manufacturing spaces.

Former FDA drug investigator, Daniel Roberts, discusses the importance of updating process validation and maintaining proper data integrity at a keynote session during INTERPHEX.

WMFTS has launched WMArchitect, a single-use product line that offers ready-to-use single-use assemblies and custom-designed workflows for biopharma fluid management.
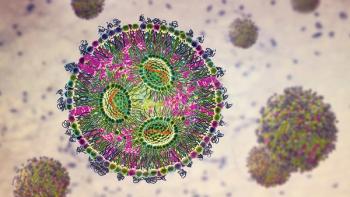
The LNP success in mRNA vaccines leads to their establishment as a standard in mRNA delivery.

Charles River has entered into separate agreements with Axovia Therapeutics and Ship of Theseus to offer plasmid DNA manufacturing services for a gene therapy and a lead candidate program, respectively.

With the launch of a new manufacturing service, Memel Biotech will offer services for discovery through to formulation for advance therapy medicinal products.

Under a collaboration agreement, Lonza will manufacture sabirnetug, Acumen Pharmaceuticals' mAb in clinical development for treating Alzheimer’s disease.