
New child-resistant packaging designs meet regulatory requirements, and consumer research into child-resistant closures continues.
Hallie Forcinio is packing editor for Pharmaceutical Technology and Pharmaceutical Technology Europe, editorhal@sbcglobal.net.

New child-resistant packaging designs meet regulatory requirements, and consumer research into child-resistant closures continues.

Packaging machines, coding equipment, inspection and data capture systems, material handling, controls, and software must work together in a serialization system.

Temperature-controlled packaging trends include prequalified systems that simplify adoption, reusable systems that are more sustainable, and new temperature-monitoring technology.

Use of temperature-controlled packaging is increasing with the growing need for cold-chain protection.

Modular design and quick-changeover features help deliver flexibility and maximize return on investment.

New packaging concepts and equipment, including systems for vial handling, printing and labeling, and parenteral packaging, were revealed at INTERPHEX 2014.

Printing, labeling, aggregation, and inspection equipment meets serialization requirements.

Displays will include new products for anticounterfeiting, labeling, and compliance packaging and new equipment for filling, aggregating, and quality control.

Using best practices for manual or automatic inspection can improve the inspection process.

Trends driving pharmaceutical packaging include product protection, productivity boosters, and patient adherence improvement.

The Drug Quality and Security Act creates national standards for serialization of drug products to protect against counterfeiting.

Capacity expansions and new products meet needs for inhaler and injector systems.

Temperature-sensitive pharmaceuticals and biologics depend on a variety of services and technologies to establish, maintain, and verify proper storage and transport conditions.
New weapons in the anticounterfeiting arsenal include authentication and labeling technologies.

Containers, components, and quality control equipment help safeguard product quality and patient safety.

Solid-dosage forms and parenteral products benefit from next-generation packaging machines.
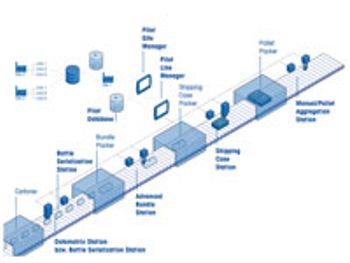
Integrated serialization systems keep pace with industry demand.

An annual Healthcare Compliance Packaging Council competition recognizes innovative pharmaceutical packages designed to improve patient adherence.

The annual INTERPHEX show presents end-to-end packaging solutions.

Bristol-Myers Squibb embarks on a multi-year journey to overcome the challenges of serialization and reap the benefits.

Equipment purchased today for the packaging line should be serialization-ready in preparation for upcoming requirements.

Medication safety and efficacy depend on maintaining products at the proper temperature.

Advances in data loggers and radio-frequency identification tags help meet the increasing need for managing the pharmaceutical cold chain.

Overt and covert packaging technologies have evolved to authenticate drugs and fight counterfeits.

Overt and covert packaging technologies evolve to authenticate drugs and fight counterfeits.

Packaging and monitoring tools protect temperature-sensitive pharmaceuticals.

Highly automated and sensitive quality-control equipment quickly identifies product faults.

Highlights included the latest in pharmaceutical packaging equipment, containers, and labels and new capabilities among contract service providers.

Our Packaging Forum editor reports on the infamous US trade show, INTERPHEX. Highlights included the latest in pharmaceutical packaging equipment, containers and labels, as well as new capabilities among contract service providers.

Visitors will see many packaging innovations at the annual industry exhibition.