
Makers of temperature-sensitive products constantly seek to ensure proper conditions during shipping and storage.
Hallie Forcinio is packing editor for Pharmaceutical Technology and Pharmaceutical Technology Europe, editorhal@sbcglobal.net.

Makers of temperature-sensitive products constantly seek to ensure proper conditions during shipping and storage.

Drug packaging performs functions such as ensuring patient well-being, providing information, preventing tampering, blocking counterfeiting, and improving compliance. Since 1977, packaging innovations have occurred in four major categories. The author provides an overview of major packaging improvements that have emerged in the past 30 years.

Exhibitors' products prevent counterfeiting, provide child resistance, protect product quality, and improve packaging-line efficiency.

Many radio frequency identification projects are moving beyond the pilot stage, supported by new hardware and software tools.

Security, the environment, aging populations, the bio-boom, and cost control are just a few of the drivers that will influence pharmaceutical packaging for the remainder of this decade.

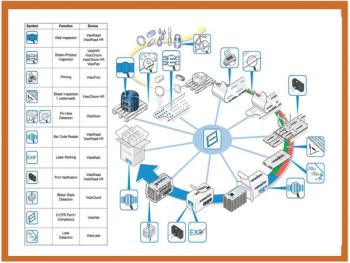
Nondestructive testing takes many forms and is gaining favor because it saves time and prevents costly product loss.

A hybrid system using paper and electronic pedigrees will be needed.

RFID is viewed by many, including FDA, as a technology with strong potential for carrying the mass serialization data needed to track and trace product and to create pedigree records.

Disappointed in progress thus far, the US Food and Drug Administration wants pharmaceutical manufacturers to make a greater effort to combat counterfeit products and recommends that they "move quickly" to implement radio-frequency identification technology.

Interphex provided an opportunity to examine the latest pharmaceutical packaging concepts and packaging machines.

Bringing Exubera to market requires extensive collaboration by Pfizer, Nektar Therapeutics, West Pharmaceutical Services Tech Group, and Bespak.

PDA's Technical Report No. 39 provides guidance for protecting temperature-sensitive products.

Although patient compliance problems have been receiving attention for at least a decade, many medications are still dispensed in bottles that contain a supply intended to last days or weeks and require considerable effort on the part of the patient or caregiver to keep track of the dosing schedule. As a result, when it comes to consistently taking the right dose at the right time for the duration of a prescription, many consumers don't do a very good job.

Cephalon partners with hardware and software providers to test item-level tagging.

Solid dosage form manufacturers have long relied on shape and color as well as on-pill imprints of logos, product names, or numbers for product identification. But in these days of heightened counterfeiting concerns, the industry has a growing interest in adding more difficult-to-duplicate features to the pool of existing product identification techniques. Added security is particularly important on high-profile or high-cost drugs, as well as on pharmaceutical products supplied in bulk for repackaging.
Most experts recommend layering protective technologies by selecting a combination of overt and covert techniques.

The unit-dose bar coding rule requires integrating many aspects of packaging design and control.

RFID and battery-powered containers are among the developments receiving attention in the pharmaceutical cold chain.

A review of Pack Expo International 2004, one of the world's largest packaging trade shows that featured the latest cutting-edge solutions.

Packaging machines for new or existing lines are easier to operate and change over than they ever were before. Today's packaging machines also accommodate a greater variety of heights, diameters, finishes, or dosage regimen counts.

Stakeholders consider ways to improve printed patient inserts.

Particulate generation and durability concerns are encouraging pharmaceutical manufacturers to seek alternatives to wood pallets.

This month's column highlights some of the latest innovations in packaging seen at this year's Interphex conference.

Comprehensive Web sites, journals, and other sources help pharmaceutical makers find packaging-related data quickly and easily.

Drug makers and their suppliers will need good planning and organization to meet the new bar coding requirements for drug products used in hospitals.

Protecting a product from temperature abuse is gaining importance as regulators seek proof of product stability throughout the distribution process.

With new features such as servo-controlled operations and modularity, today's packaging machines offer enhanced flexibility, increased uptime, and reduced product waste.

The process of designing a new drug package must follow specific steps to ensure low costs and rapid market approval.

New blister packaging offers patient compliance aids, child-resistant mechanisms, and space to include brand information.

Interphex provided pharmaceutical manufacturers an opportunity to view the latest packaging materials and machinery.