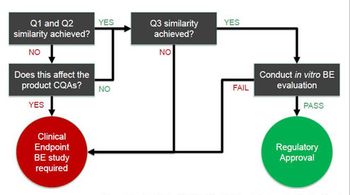
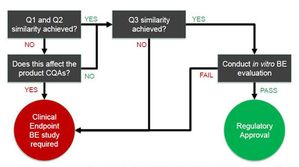
This article examines IVBE testing requirements for topical creams and explores some of the analytical techniques necessary.
Jennifer Burt is applications specialist at Malvern Panalytical.

This article examines IVBE testing requirements for topical creams and explores some of the analytical techniques necessary.
Published: September 1st 2018 | Updated: