Pharmaceutical Technology Editors
Articles by Pharmaceutical Technology Editors

GE Healthcare, the health business of General Electric, will dedicate $1 billion of its total R&D budget during the next five years to its technologies for manufacturing biopharmaceuticals and for cancer research. Part of the money will go toward expanding the company's cancer-diagnostic and molecular-imaging capabilities, as well.

Bristol-Myers Squibb Completes Acquisition of Amira Pharmaceuticals; PPD Appoints Raymond H. Hill as CEO; and More.

Pfizer plans to invest EUR 145 million ($200 million) in its Grange Castle biotech-manufacturing site. Meanwhile, completion of the merger between Alkermes, Inc. and Elan Drug Technologies was announced in a press release on Sept. 16, 2011, following the approval by Alkermes, Inc. shareholders on Sept. 8, 2011.

In a 2004 guidance, FDA says that the "use of redundant sterilizing filters should be considered in many cases." But not all manufacturers agree on what redundant filtration is.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the August 2011 edition from Lechler and National Bulk Equipment.
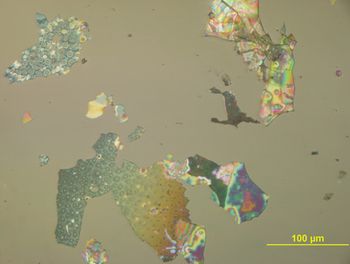
Glass flaking or delamination can result in a failed quality-assurance test, thus bringing production to a halt and causing substantial revenue loss. If glass delamination remains undiscovered, it can pose a serious contamination risk to the drug product and a potential health risk to the public.

In our manufacturing process, we are running into issues with our vial stoppers clumping in the feeder bowl. How can we ensure that the stoppers go through smoothly without clumping up?

AmerisourceBergen Completes the Acquisition of IntrinsiQ; Xcelience Expands its Manufacturing and Packaging Services; and More.

The International Society for Pharmaceutical Engineering will soon publish an update for its guide to sterile-product manufacturing facilities. The new publication will replace the original guide and contain practical information about technological advances in sterile manufacturing.

The UK's Technology Strategy Board has partnered with the Engineering and Physical Sciences Research Council to invest up to £9 million ($14.2 million) in grant funding with the aim of driving proof-of-concept ideas to pilot technology demonstration for healthcare.

FDA Makes Organizational Changes Within CDER.

GSK, Anacor Expand R&D Collaboration; Xoma Announces Management Changes; and More.

USP Expands Presence in India.

A roundup of developments in corporate social responsibility and sustainability from the bio/pharmaceutical industry, its suppliers, and other public and private organizations.

Valeant Agrees to Acquire Afexa Life Sciences; Merck KGaA Names Robert Yates President of Millipore Division; and More.

FDA published a draft guidance that lists recommendations to follow, data to provide, and criteria to meet and describe in new drug applications and abbreviated new drug applications for scored tablets.

EMA announced that a new version of the validation criteria for electronic applications for human medicines comes into effect on Sept. 1, 2011.

African Community Joins ICH's Steering Committee.

The UK's Medicines and Healthcare products Regulatory Agency (MHRA) issued a number of press releases on Aug. 25–26, 2011, regarding the discovery of rogue medicines in packs of Reckitt Benckiser's Nurofen Plus.

Last week, Ben Venue Laboratories decided to exit the contract-manufacturing business during the next several years, thus ending more than 70 years of service in this field. To ensure the supply of medically necessary products, the company will work with its customers to develop and execute long-term transition plans.

Eisai Establishes New Pharma Sales Subsidiary in Mexico; Cangene Names John A. Sedor as President and CEO; and More.

The UK government announced that a record £800 million ($1.32 billion) in funding would be set aside for translational research to boost the development of medicines, treatments, and care for patients, particularly in the fields of cancer, diabetes, and heart disease.

Xcelience Expands its Facility in Tampa, Fla.; Delta Pharma Names John Ebeid as VP of Outsourcing; and More.

FDA, Arkansas Sign Agreement to Enhance Regulatory Science.

PhRMA has released a statement expressing their opposition to laws that would alter Medicare Part D.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the August 2011 edition from Chemineer and IDS.

Achieving a consistent level of quality control could greatly reduce waste and save money for the pharmaceutical industry. But why has talk about Six Sigma died down at a time when it could be of great benefit?

FDA Issues Final Guidance Regarding cGMPs for PET Drugs.

EMA has concluded in a recent report that a pilot program investigating the mutual benefits of joint international inspections of API manufacturing facilities has been a success. FDA has also reached a similar conclusion based on the findings.

Catalent Expands Global Cold-Chain Supply Operations; Nycomed US Names Brian A. Markison as CEO; and More.



