Pharmaceutical Technology Editors
Articles by Pharmaceutical Technology Editors

Two popular methods for detecting protein aggregates are analytical ultracentrifugation (AUC) and size-exclusion chromatography?multiangle light scattering (SEC?MALS). These techniques? results correlate relatively well, but each one has its own strengths.

AstraZeneca agreed to settle a sex-discrimination lawsuit by paying $250,000 to 124 women who worked at the company's Philadelphia Business Center.

Aveo Forms Drug-Development Pact with Centocor Ortho Biotech; Generic Pharmaceutical Association Appoints Jim Fenton as Senior Vice-President of Government Affairs; and More.

FDA Issues Final Guidance to Amend IND Reporting Requirements.

A roundup of developments in corporate social responsibility and sustainability from the bio/pharmaceutical industry, its suppliers, and other public and private organizations.

An independent report released by the European Medicines Agency highlighted a number of recommendations to aid the agency in its communication of the benefits and risks of medicines.

FDA added a searchable database of inspection data to its website. The database lists the names and addresses of facilities that the agency inspected during fiscal years 2009 and 2010.

Valeant agrees to acquire Sanitas Group; Geron makes several senior appointments; and More.

The greatest challenge faced by the EMA is the continuing expansion of the EU.

FDA held a joint meeting of its Nonprescription Drugs Advisory Committee and Pediatric Advisory Committee to discuss whether new dosing information for oral over-the-counter drug products containing acetaminophen should be added to the label for children under age 2.

Almac opens new North American headquarters; Sanofi Pasteur appoints Damian Braga as senior vice-president of global commercial operations; and More.

Global spending on medicines will total almost $1.1 trillion by 2015, according to an IMS Institute for Healthcare Informatics study.

Teva Pharmaceutical Industries agreed to pay shareholders $460 million in cash to acquire a 57% stake in Taiyo Pharmaceutical Industry. Teva also will offer to buy all outstanding shares of Taiyo.

AstraZeneca, GlaxoSmithKline, and the University of Manchester form new research collaboration for inflammation; BASi names Michael Zhou as senior director of R&D; and More.

EMA is working with its European and international regulatory partners to monitor and evaluate ?the possible risk of radioactive contamination of medicines manufactured in Japan following the radiation leak from the Fukushima Daiichi nuclear power plant.?

Recent recalls, including that of American Regent?s caffeine and sodium benzoate injection on May 5, 2011, highlight the importance of particulate inspection, and they might lead observers to ask whether current inspection methods are sufficiently effective.

How do you assign a minimum sample weight for a US Pharmacopeia <41> balance application when the tested repeatability gives a standard deviation of zero?
PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the May 2011 edition from David Round Company and Globe Medical Tech.

Filling active ingredients directly into capsules is probably the quickest option for entering clinical trials. This case study compares manual and automated methods of capsule filling.

In fiscal year 2010, the number of patent settlements in which the manufacturers of branded products paid makers of generic drugs to postpone the introduction of their products reached its highest level ever, according to the Federal Trade Commission.

FDA is asking for input on the development of a user-fee program for biosimilar and interchangeable biological product applications.

INC Research agrees to acquire Kendle; CMC Biologics hires Claes Glassell as CEO; and More.

The IPEC is soliciting public comment about a draft plan for the independent certification of manufacturers and suppliers of pharmaceutical excipients.

Velesco partners with Vindonwestech; NeurogesX announces retirement of CEO Anthony DiTonno; and More.

Israel-based Teva Industries seals deal to acquire Cephalon for $6.8 billion.
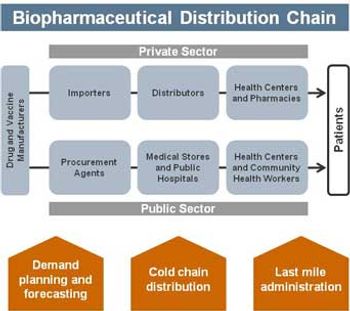
This article, which focuses on distribution and administration, is Part III of a three-part series on biopharmaceutical issues in public health, government, and developing-world markets.

A roundup of developments in corporate social responsibility and sustainability from the bio/pharmaceutical industry, its suppliers, and other public and private organizations.
Efforts are made to educate health workers in less developed countries.

sanofi-aventis signs research pact with Stanford; Paul Maffuid joins AAIPharma Services; and More.

Warner Chilcott announced in a press release on Apr. 18, 2011, its intentions restructure, placing 500 Western European jobs on the line.




