
McNeil-PPC Lowers Maximum Daily Dose for Single-Ingredient Acetaminophen Products Sold in the US; Sanofi Names David-Alexandre Gros as Chief Strategy Officer; and More.

McNeil-PPC Lowers Maximum Daily Dose for Single-Ingredient Acetaminophen Products Sold in the US; Sanofi Names David-Alexandre Gros as Chief Strategy Officer; and More.

The International Society for Pharmaceutical Engineering published a guidance document that defines current best practices in pharmaceutical manufacturing applications for handling gases that come into direct contact with the biopharmaceutical and pharmaceutical process steams.

FDA Website Provides Information on Drug Shortages.

A roundup of developments in corporate social responsibility and sustainability from the bio/pharmaceutical industry, its suppliers, and other public and private organizations.

AstraZeneca Receives FDA Approval for Brilinta; Sanofi Makes Several Senior Appointments; and More.

The global production of seasonal influenza vaccine will double to 1.7 billion doses by 2015, according to a World Health Organization presentation.

According to a price report by Medecins Sans Frontieres (MSF, or Doctors Without Borders) released July 18, 2011, several pharmaceutical companies have withdrawn drug price reductions in so-called middle-income countries, such as India, Indonesia, Thailand, Vietnam, Ukraine, Colombia and Brazil.

Amgen Resolves Patent Dispute with Teva; PharmaNet Development Group Names George Scott Vice-President of Bioanalytical Services; and More.

FDA Approves the Influenza Vaccine Formulation for the 2011-2012 Flu Season.

The pharmaceutical industry and US regulatory bodies have not responded adequately to the increasing level of outsourced manufacturing in countries such as China and India, according to a new white paper by the PEW Health Group.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the July 2011 edition from BETE Fog Nozzle and Continental Disc.

FDA banned the importation of products manufactured at the Mexican unit of Dr. Reddy's Laboratories. The import ban is a result of the company's failure to correct the violations listed in a recent Warning Letter to the agency's satisfaction.

Sanofi Forms Research Pact with Weill Cornell Medical Center; Steven A. Nichtberger Resigns as President and CEO of Tengion; and More.

A roundup of developments in corporate social responsibility and sustainability from the bio/pharmaceutical industry, its suppliers, and other public and private organizations.

The International Federation of Pharmaceutical Manufacturers and Associations issues a Framework for Action for noncommunicable diseases.

The European Medicines Agency has responded positively to a new directive published in the Official Journal of the European Union addressing concerns over increases in falsified medicines in the supply chain.

Sanofi Forms Research Pact with Weill Cornell Medical Center; Steven A. Nichtberger Resigns as President and CEO of Tengion; and More.

FDA Publishes Final Guidance on Dissolution Testing.

Roche Establishes R&D Institute in France; Sanofi Appoints Peter Guenter as Senior Vice-President, Europe; and More.

President Obama unveiled an Advanced Manufacturing Partnership designed to reinvigorate the country's manufacturing sector.

The European Medicines Agency (EMA) has held a second forum regarding the implementation of new pharmacovigilance legislation, which gave stakeholders the opportunity to discuss their expectations on various aspects of the new legislation's execution.

FDA Issues Warning Letter to Dr. Reddy's Following Inspection of the Company's Mexico-Based API Manufacturing Plant.

The traditional method of conveying information in the brief summary of a printed prescription-drug advertisement is neither the most comprehensible nor the most preferred by consumers, according to an FDA study.

Array BioPharma Restructures to Focus on Development of Clinical Programs; AstraZenica Plans Russian Expansion; and More.

Biogen Idec Receives Approval for the Avonex Pen; Xceleron Makes Several Senior Appointments; and More.

FDA Issues Consent Decree of Condemnation, Forfeiture, and Permanent injunction Against H&P Industries, the Triad Group, and Three Individuals.

After looking back at the first year of its Bad Ad outreach program, FDA judged that the initiative has successfully raised awareness about misleading promotion, according to an FDA press release.
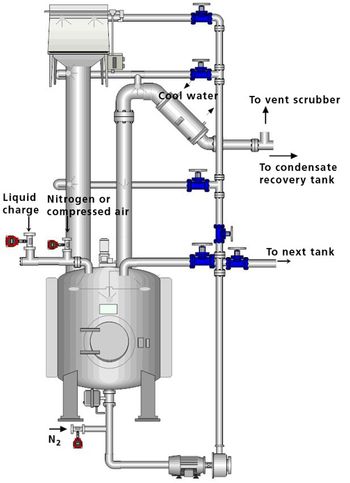
The author describes the benefits and challenges inherent to cleaning in place (CIP). The article also describes the development and validation of a CIP cycle.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the June 2011 edition from Meissner and Telstar.

Our company is getting low and unpredictable cycle life out of our diaphragm valves. This problem has caused us to implement standard operating procedures for frequent replacement of the diaphragms, which is costly and time consuming. What could be happening, and what are our options?