
Also, FDA extended the deadline for the pilot program for the submission of CMC information.

Also, FDA extended the deadline for the pilot program for the submission of CMC information.

Company and People Notes: Wyeth and Ambrx form development pact; Elite Pharma appoints CEO and CSO; more...

The US Food and Drug Administration sent Bayer Schering Pharma (Berlin) a Warning Letter on Aug. 5, 2009, citing deviations from current good manufacturing practice in the manufacture of nonsterile active pharmaceutical ingredients (APIs).

Vaccine makers and other pharmaceutical manufacturers using yeast protein-expression systems are taking note of a discovery this week by a team of researchers who have found RNA interference in Saccharomyces castellii.

Also, FDA's enforcement of its rules for response times for Form 483s went into effect this week, more...

Eli Lilly and Company (Indianapolis, IN) is undergoing a companywide reorganization that is intended to accelerate the progress of the company's pipeline.

Company and People Notes: Sanofi Pasteur signs H1N1 vaccine deal with Brazilian government; Helsinn Group appoints CEO of US business; more...

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the September 2009 edition from Gore and Oystar.

Small-format packaging equipment can provide benefits to the pharmaceutical industry such as quick changeover and low tooling costs. The machines also can shorten the time it takes to bring a product to the market.

Investing time and money in auditing and optimizing a steam system can pay off quickly, especially because the costs of energy, maintenance, and downtime are steadily rising.

More than 80% of pharmaceutical and biologics companies are completing their postmarketing studies and regulatory obligations in a timely manner.

Generic-drug companies are increasingly viewing the development of controlled-release formulations as a way of obtaining a competitive edge, according to a report published by Espicom Business Intelligence in late August 2009.

Company and People Notes: Neoprobe and Laureate Pharma form manufacturing agreement; Akela Pharma appoints CEO and chairman; more...

The US Pharmacopeial Convention (USP) and the Permanent Commission of the Pharmacopeia of the United Mexican States (FEUM) signed a memorandum of understanding (MOU) last week.

Also, Orexo and Novartis form agreement; Affitech appoints Robert Burns CEO; more...

The US Food and Drug Administration last week issued the final draft of its guidance for industry titled Labeling of Nonprescription Human Drug Products Marketed Without an Approved Application as Required by the Dietary Supplement and Nonprescription Drug Consumer Protection Act: Questions and Answers.

Also, FDA issues several recent enforcement letters to Cambrex, Johnson & Johnson, and Pedinol.

An outstanding new book reviews alternative solvents with an eye to sustainable pharmaceutical processes.

A second-generation and green manufacturing process for testosterone provided economic and ecological benefits.
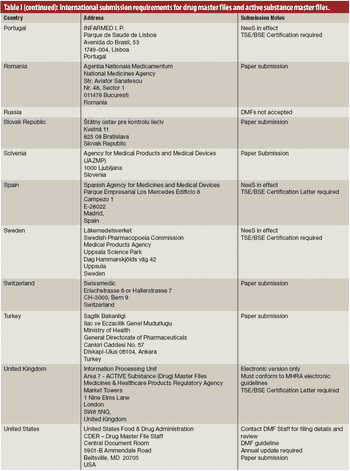
The author describes several issues in creating drug master files and active substance files for active pharmaceutical ingredients and intermediates.

On Aug. 19, 2009, the US Food and Drug Administration opened a new Center for Tobacco Products on the agency's White Oak Campus in Silver Spring, Maryland.

Scientists have modified a tobacco plant to produce a vaccine for norovirus, the viral infection sometimes called the "cruise ship virus."

Company and People Notes: UCB and Novartis form agreement; AAIPharma appoints VP of regulatory affairs; more...

Also, USP signed a Memorandum of Understanding with the Permanent Commission of the Pharmacopeia of the United Mexican States, more...

Researchers at the University of Illinois (Champaign) have developed a cancer drug delivery system that reportedly kills target tumor cells, spars healthy cells, and has effects that can be reversed to halt potentially hazardous side effects.

Also, FDA publishes draft guidances of two ICH Annexes; EMEA sets format for compliance advice; more...

India accounts for approximately 3% of the global outsourcing market, which indicates a significant opportunity for growth, according to a report published by Ernst and Young and the Organization of Pharmaceutical Producers of India.

Also, XCELERON and GSK form agreement; Millipore appoints VP of life sciences; more...

The US Food and Drug Administration?s Draft Guidance for Industry?Process Validation: General Principles and Practices provides a life-cycle approach for validating pharmaceutical processes and aims to help pharmaceutical companies achieve consistently high product quality.
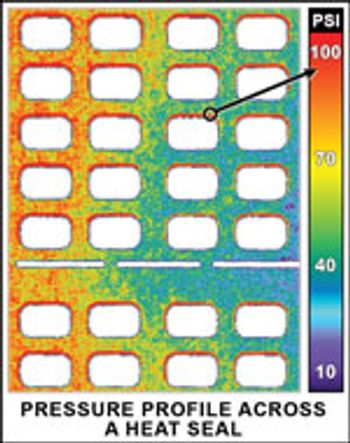
PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the August 2009 edition from AEC and Sensor Products.