PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the May 2009 edition from Continental Disc and Filimatic.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the May 2009 edition from Continental Disc and Filimatic.

On May 7, 2009, as part of the President's fiscal year (FY) 2010 budget, the US Food and Drug Administration requested a budget of $3.2 billion, which is 19% more than the agency's current budget.

Also, Takeda to consolidate operations in Ireland; FDA to redesign website; Archemix names president and CEO; more...

Also, Oxford BioMedica forms collaboration with sanofi-aventis; FDA requires labeling changes for botulinum toxin producs; C. Richter King joins IAVI; more...

This week, pharmaceutical industry regulators and manufacturers moved quickly to address public health concerns regarding the outbreak and spread of H1N1 virus infection (swine flu). The following is an overview of key developments.

Pharmaceutical companies must make bold moves and "step outside of their sector" if they are to survive.

A novel cleanroom apparel design incorporates modern concepts to help minimize contamination. Take a tour of the design.

Also, Roche's Avastin trial does not meet endpoint; Innate Pharma names regulatory VP; more...

Senators Chuck Grassley (R-IA) and Ted Kennedy (D-MA) introduced legislation last Thursday that would give the US Food and Drug Administration more resources to inspect domestic and foreign plants that manufacture drugs and medical devices.

The Pharmaceutical Research and Manufacturers of America released a statement this week in response to recent media reports regarding the amount of pharmaceutical ingredients being discharged by manufacturing facilities into the environment.

The US Food and Drug Administration finalized a Guidance for Industry this week that aims to clarify the submission of new drug applications (NDAs) and biologics license applicants (BLAs) using the common technical document (CTD) format, including the electronic CTD (eCTD).

On April 2, the Health Products and Food Branch of Health Canada and EU (consisting of the European Commission [EC] and European Medicines Agency [EMEA]) released "Implementation Plan for Regulatory Cooperation on Medicinal Products."

Last week, Johns Hopkins Medicine (JHM) adopted a new policy to protect patients by limiting the influence of pharmaceutical marketing on faculty and physicians? decisions.

Also, Sanofi-aventis acquires Medley and Laboratorios Kendrick; Eli Lilly's Cook to retire from board; more...

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the April 2009 edition from Buzdar and Kaeser.

ATSM and ICH approaches, in place of traditional qualification or integrated commissioning and qualification (C&Q) using impact assessment, can make projects and processes more efficient and help facility owners and designers ensure compliance, quality, and safety when defining acceptance criteria for their critical process systems and equipment.

Many drugmakers have begun to evaluate and improve their manufacturing operations to become more economically and environmentally sustainable.

The Pharmaceutical Research and Manufacturers of America (PhRMA) elected David Brennan, CEO of AstraZeneca, as board chairman.

Also, Genzyme and Bayer HealthCare form agreement; FDA releases draft guidances; TransMolecular appointed Robert Radie president and CEO

Senator Charles Grassley (R-IA) sent a letter to Frank Torti, acting commissioner of the US Food and Drug Administration, to express concern about a memo that Torti sent to agency staff.

Also, SOCMA changes name; two FDA approvals; Biogen Idec names chief operating officer; more...

On March 26, the Michigan House of Representatives passed House Bill 4316, effectively repealing part of a 1996 law that provides drug companies immunity from liability lawsuits involving products that have been approved by the US Food and Drug Administration.

In a recent book, UK regulators explain how to establish a pharmacovigilance system.

Also, Hospira to reduce workforce; WuXi AppTech makes senior appointments; more...

In a case decided on Mar. 20, 2009, the US Court of Appeals for the Federal Circuit invalidated a US Patent and Trademark Office (PTO) Final Rule that governed the number of applications that parties may file to seek continued examinations of patent applications.

The US Pharmacopeial Convention (USP) and the National Institute for the Control of Pharmaceutical Biological Products (NICPBP), China's agency for overseeing the quality of large- and small-molecule drugs, signed a memorandum of understanding (MOU) to bolster the quality of medicines in China and in the countries that buy Chinese drug products, including the United States.

Also, Genzyme receives warning letter; Mesa Laboratories appoints John J. Sullivan CEO and a member of the board of directors; more...
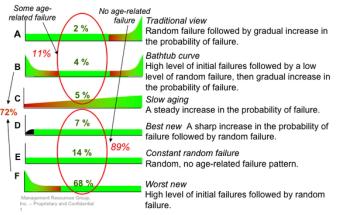
Time-based maintenance programs preserve the equipment but usually not its function, and they do not mitigate equipment failure for the balance of a machine's life cycle.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the February 2009 edition from PortaFab and Sterling.

The spotlight on the biopharmaceutical industry is intensifying, as recently evidenced by Pfizer's (New York) ongoing acquisition of Wyeth (Madison, NJ), which was initiated partly to reduce the former's dependence on small-molecule drugs.