
Cooling water is a critical component in the research and development, bulk manufacturing, and packaging of pharmaceuticals.

Cooling water is a critical component in the research and development, bulk manufacturing, and packaging of pharmaceuticals.

Also: DSM's North Carolina facility receives SafeBridge certification; Dynavax CFO to retire; more...

On August 6, the Biotechnology Industry Organization (BIO) filed an amicus brief asking the US Supreme Court to overturn Bilski v. Doll, a decision of the US Court of Appeals for the Federal Circuit.

The US Food and Drug Administration released its Guidance for Industry titled Pharmaceutical Components at Risk for Melamine Contamination.

Also, EMEA gives public access to GMP data; SOCMA speaks out on toxic substances; more...

Also, Pfizer forms two research agreements in China; NanoInk appoints John Kubricky to its scientific advisory board; more...

The Energy and Commerce Committee of the US House of Representatives approved H.R. 3200, America's Affordable Health Choices Act, last Friday.

The US Food and Drug Administration and European Medicines Agency (EMEA) launched this week a joint initiative to collaborate on international good clinical practice (GCP) inspection activities.

The author of an ambitious book about quality control falls short of reaching his goals.

The author of a book about biopharmaceutical production includes irrelevant information.

The authors explain waivers and deferrals for pediatric studies of drugs and biologics as provided by the Pediatric Research Equity Act of 2007.

On July 18 in Mumbai, Hillary Rodham Clinton's private, cosy tete-a-tete with 10 of India Inc's most sought-after billionaires, was a power breakfast the likes of which the city's corporate czars had not seen in a long time.

Researchers working on extending the shelf life of antibody drugs may find help in a computer model developed by a research team at MIT (Cambridge, MA).

This week, Sanofi Pasteur, the vaccines division of sanofi aventis (Paris), acquired the ShanH subsidiary of Mérieux Alliance, a holding company.

Also, Isogen completes sterile facility; Dishman appoints head of generic API business; FDA releases final rule on authorized generic drugs and NDA submissions; more...

Last week, Rep. George Miller (D-CA), Rep. Charles Rangel (D-NY), and Rep. Henry Waxman (D-CA), introduced the America's Affordable Health Choices Act (H.R. 3200) to reform the nation's healthcare system.

The US Food and Drug Administration last week posted the final version of its Guidance for Industry, ANDAs: Impurities in Drug Substances.

Also, Gilead and Tibotec form agreement; FDA approves 2009-2010 flu vaccine; Catalent appoints VP in packaging unit; more...

Devices that measure relative humidity (e.g., sensors and transmitters) play a relatively small role in cleanroom management, but their failure can cause significant problems. Operators should bear several factors in mind to ensure that sensors function properly and maintain the appropriate humidity.

Drugmakers have the common goal of manufacturing safe and sterile pharmaceutical products and understand that the filtration process is a critical means of achieving this goal. Preuse filter-integrity testing provides evidence that a filter will perform correctly, has the right pore size, and has been installed correctly.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the July 2009 edition from Mettler Toledo Safeline and Munters.

Also, Lonza and Medarex sign agreement; Covance appoints VP and chief scientific officer of global analytical services; more...

In the wake of Dow Chemical?s announcement that it is to close facilities in the UK, the country?s biggest union, Unite, has called for urgent intervention from the Government to avert, what it believes, could be a crisis in the UK chemical sector.

Last week, the House of Representatives passed H.R. 2997, the Fiscal Year 2010 Agriculture?FDA Appropriations bill, which sets aside $2.995 billion for the US Food and Drug Administration.

This week, the Biotechnology Industry Organization (BIO) and the Generic Pharmaceutical Association (GPhA) issued their opposing statements in reaction the July 13 passage of an amendment from the US Senate Committee on Health, Education, Labor, and Pensions (HELP) regarding the creation of a pathway for the approval of new competitors of biologic drugs.

Also, Lundbeck acquired LifeHealth; FDA is seeking a director of its new tobacco regulation branch; Charles River Labs announces personnel changes; more...

The US Pharmacopeia is revising its monographs for four pharmaceutical excipients: propylene glycol, sorbitol solution, sorbitol sorbitan solution, and noncrystalllizing sorbitol solution.

The Federal Coordinating Council for Comparative Effectiveness Research recommended that data infrastructure be the primary investment for the US Department of Health and Human Services's (HHS) comparative-effectiveness research (CER) funds.
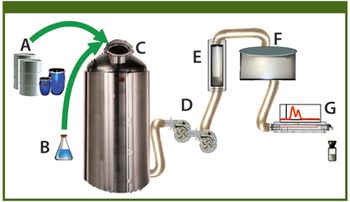
The author provides a review of PAT and tools such as near infrared analysis that may facilitate the use of PAT in the biopharmaceutical sector.

On June 25, US Marshalls seized all drug products and ingredients at three facilities of Caraco Pharmaceutical Laboratories.