PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the November 2008 edition from ResistoPure and Millipore.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the November 2008 edition from ResistoPure and Millipore.

Until recently, automated inspection of solid-dose pharmaceuticals was crude, expensive, slow, and difficult to change over. But technological advances have ushered in a new class of vision-inspection systems that is more effective and more commercially viable than older systems.

Also, Sartorius Stedim Biotech GmbH to acquire Wave Biotech; AstraZeneca's John Patterson to retire; more...

Arguments in the Supreme Court case Wyeth v. Levine have concluded, leaving both sides to wait possibly until early 2009 for a decision in a preemption case involving the misadministration of Wyeth's "Phenergan."

The US Food and Drug Administration sent Warning Letters to Bayer HealthCare (Morristown, NJ) about the company's "Bayer Women?s Low Dose Aspirin + Calcium" and "Bayer Aspirin with Heart Advantage" over-the-counter (OTC) products.

Brief pharmaceutical news items for November 2008.

IPEC Chairman Dave Schoneker discusses current efforts toward facilitating regulatory reviews of new excipients.

Scott Sutton discusses the current state of USP ‹1117› and USP's plans for future revisions.

Pfizer and Lilly recently resolved issues related to their marketing practices for certain products.

Also, Maxygen looks to costs, jobs; Receptor BioLogix appoints Dale R. Pfost CEO; more...

Pfizer and UCB formed a technology company named Cyclofluidic with the aim of accelerating the drug-discovery process.

The US Food and Drug Administration released a draft guidance that reviews the agency's plan to offer priority-review vouchers to companies developing new treatments for neglected tropical diseases.

Rep. John D. Dingell (D-MI), chairman of the US House of Representatives Committee on Energy and Commerce, and Rep. Bart Stupak (D-MI), chairman of the Oversight and Investigations Subcommittee, sent letters to the US Food and Drug Administration, Shaw Science Partners, and EthicAd to request information about a new FDA website.

Also, MedImmune opens Cambridge, UK, facility and makes reverse engineering pact with Omninvest; BD Medicine appoints Carol Adiletto VP of clinical and regulatory affairs; more...

Also, Millipore opens new membrane-casting manufacturing facility in Ireland; Surface Logix appoints Keith Dionne president, CEO, and a member of the board; more...

According to a July 11, 2008 memorandum posted by the Center for Biologics Evaluation and Research, starting Oct. 15, Health Level 7 structured product labeling in XML (extensible markup language) will be the only acceptable presentation in electronic format for the submission of content of labeling that CBER can process, review, and archive.

At a public meeting, the Medicare Payment Advisory Commission (MedPAC) discussed its recommendation that Congress establish a national database to publicly reveal financial relationships between physicians and the pharmaceutical industry.

Integrating MES with enterprise resource planning (ERP) systems can bring companies greater control over production than before and make manufacturing processes more flexible.

Under a definitive merger agreement, Eli Lilly and Company will acquire ImClone Systems, Inc. (New York) in a cash tender offer of approximately $6.5 billion.

Rep. John D. Dingell (D-MI), chairman of the US House of Representatives Committee on Energy and Commerce, and Rep. Bart Stupak (D-MI), the chairman of the Oversight and Investigations Subcommittee, sent a letter to the US Department of Health and Human Services (HHS), questioning the US Food and Drug Administration?s use of agency resources to hire an outside public-relations firm to create a positive public image of the agency.

Also, Merck & Co. discontinues development of its obesity drug taranabant; Synthetech names Frederic Farkas director of manufacturing; more...
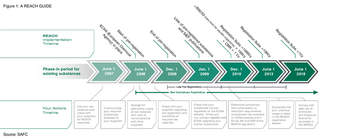
The pharmaceutical industry faces compliance with the REACH regulation, the European Union's regulation on chemicals and their safe use. Fully understanding the requirements, achieving compliance, and developing strategies for working with suppliers are key for avoiding interruption of the supply chain.

Brief pharmaceutical news items for October 2008.

Also, Alpharma advises shareholders to reject King's offer; ImClone rejects raised BMS offer; Immunogen appoints Daniel M. Junius, more...

The International Electrotechnical Commission (IEC) has approved HART 7.1, the WirelessHART Communication Specification, as a Publicly Available Specification (IEC/PAS 62591Ed.1). WirelessHART is the first industrial wireless communication technology to achieve this level of approval.

Eli Lilly and Company plans to launch an online registry of its payments to physicians in 2009.

Ionic liquids provide an alternative to certain solvents in select reactions used to synthesize intermediates and active pharmaceutical ingredients.

Also, Zentiva accepts Sanofi's increased takeover bid, Oriel Therapeutics appoints Richard Fuller CEO, more...

On November 3, the US Supreme Court will hear the case of Wyeth v. Levine, in which the drug company has challenged the ruling of a Vermont Supreme Court decision to award Diana Levine $6.8 million.

A new study concludes that an approval pathway for affordable follow-on biologics should be based on the Hatch–Waxman Act of 1984.