
Albany Molecular Research, Affymax, Antares Pharma, AstraZeneca, Brookwood Pharma, DelSite, Immtech, Nektar, VaxGen, Vical

Albany Molecular Research, Affymax, Antares Pharma, AstraZeneca, Brookwood Pharma, DelSite, Immtech, Nektar, VaxGen, Vical

The UK Medicines and Healthcare Products Regulatory Agency (MHRA, London) has reissued its recall of a specific batch of counterfeit ?Lipitor? 20-mg tablets. MHRA, in conjunction with Pfizer (New York City, NY), first issued the recall of batch number 004405K1 in July 2005. The new recall is in response to the discovery of more packages of the counterfeit drug in the United Kingdom.

Roxane Laboratories (Columbus, OH), a Boehringer Ingelheim company, is conducting a voluntary recall in the United States and Puerto Rico of a single manufacturing lot of "Azathioprine"tablets, USP 50 mg.

Antigenics, Gilead, Inyx, KVD Pharma, MedImmune, Novartis, Solvias

On July 18, the US Food and Drug Administration posted a Warning Letter issued Watson Laboratories Caribe, Inc. (Corona, CA) by FDA's San Juan (PR) district office.

Seeking more than $5.8 million in damages and the recovery of nearly $1.8 billion in punitive damages, RxUSA Wholesale (Port Washington, NY) filed a complaint against 16 major US pharmaceutical manufacturers and 5 drug wholesalers.

Vincent L. Vilker, Ph.D., has been appointed director of FDA's Office of Testing and Research (OTR). Vilker was formerly chief of the Biotechnology Division at the National Institute of Standards.

The US Food and Drug Administration today posted its final "Guidance for Industry: Providing Regulatory Submissions to the Center for Biologics Evaluation and Research (CBER) in Electronic Format: Lot Release Protocols."

AAI Pharma, Alexion Pharmaceuticals, Altana, Athenagen, Cardinal Health, Nektar, Roche

A group of researchers from Georgia Institute of Technology (Atlanta, GA) are using high-throughput ionization techniques to identify and measure the ingredients in counterfeit drugs.

Late last week, Pliva d.d. (Zagreb, Croatia) announced that the US Food and Drug Administration had granted final approval for its warfarin sodium tablets and azithromycin for oral suspension.

The US Food and Drug Administration (Rockville, MD) announced that Baxter Healthcare Corp. (Deerfield, IL) signed a consent decree relating to the company's "Colleague" volumetric infusion pump and "Syndeo" patient-controlled analgesic syringe pump.

Vaccine maker Sanofi Pasteur, Inc. received a US Food and Drug Administration Warning Letter, dated June 30, citing "significant deviations" from current good manufacturing practices in the production of monovalent concentrates used in the company?s ?Fluzone? influenza vaccine.

Abbott Labs, Cambrex, Cardinal Health, Charles Ross and Son Co., Janssen, West Pharmaceutical Services

Does the competition from authorized generics really help lower drug prices and boost healthcare savings?
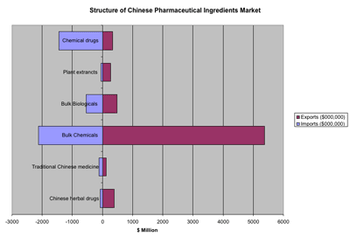
The sixth CPhI China exhibition, presented June 27?29 in Shanghai, offered a showcase for the explosive growth of the Chinese pharmaceutical sector.

University of Buffalo (Buffalo, NY, www.buffalo.edu) researchers have developed a drug delivery system that uses an external magnetic field to guide drug-filled nanocarriers to cultured tumor cells.

Biopharmaceutical company Lipoxen PLC (London, UK) has developed a Hepatitis E vaccine using its novel vaccine delivery technology "ImuXen," which the company claims to be easy to manufacture. According to the company, the proprietary liposomal formulation method delivers vaccine materials to the immune system in a manner designed to emulate the response of a natural encounter with the infection agent.

Pharmaceutical Science & Technology News

Last week, the Generic Pharmaceutical Association (GPhA Arlington, VA) praised a proposal by the Senate Agricultural Appropriations Subcommittee that, if approved, would provide $10 million in additional funding for the US Food and Drug Administration?s (Rockville, MD) Office of Generic Drugs.

Baxa Corporation, Eli Lilly, PLIVA, SkyePharma, Wyeth

The US Food and Drug Administration (Rockville, MD) is restructuring its Office of Regulatory Affairs (ORA) into two offices.

On June 15, 2006, the US Food and Drug Evaluation?s Center for Drug Evaluation and Research (Rockville, MD) issued a 7-page warning letter to Ranbaxy Laboratories (Himachal Pradesh, India) for violations to US current good manufacturing practices.

Coinciding with the 100th anniversary of the US Food and Drug Administration (Rockville, MD), Representative Henry A. Waxman (D-CA) released a statement this week expressing his concern over ?an agency that is adrift and floundering.?

This week, the US Food and Drug Administration (Rockville, MD) posted a Warning Letter issued May 31 by its New Jersey District Office (Parsippany, NJ) to Neil Laboratories (East Windsor, NJ) for CGMP deviations.

Drug development opportunities, specification development, and new vaccine technologies were highlighted at the AAPS National Biotechnolgy Conference in Boston this week. More than 1100 attendees from 19 countries participated in the event.

Microsoft Corporation (Redmond, WA) announced the winners of the Microsoft Pharmaceuticals and Life Sciences Innovation Awards 2006 at this year?s meeting of the Drug Information Association in Philadelphia, Pennsylvania. A four-person panel of industry experts selected winners for the innovative use of Microsoft products in pharmaceutical and life sciences business processes and practices.

Avant, Barr, BASF, Degussa, Laureate Pharma, Millennnium Pharmaceuticals, Northfield Labs

Alpharma, Bayer, MicroTest, NexMed, Penwest

This week?s PharmTech Annual Event (www.pharmtechevent.com) in Somerset, New Jersey, targeted approaches to improving drug development and quality through optimizing processes, managing risk, and controlling variations in manufacturing operations.