
Letter to the Editor: Glen Jon Smith

Letter to the Editor: Glen Jon Smith
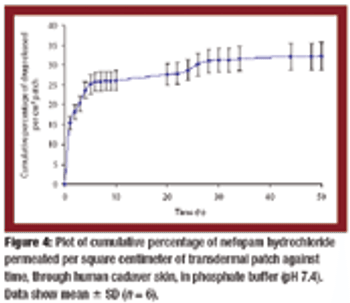
Transdermal matrix-type patches of Nefopam hydrochloride with a combination of pressure-sensitive adhesives were developed. The polymeric composition provided a controlled and sustained release of the drug from the patches and demonstrated favorable physicochemical characteristics.

FDA Recommends Changes for 2006-2007 Flu Vaccine

Drug Sales Up 5.4%...But Growth Rate Slowing

Warning Letter: Southern Meds

Baxter Wins Contract to Develop Cell-Based H5N1 Vaccine

GPhA Seeks Additional ODG Funding, Not User Fees

Industrial Info Resources reports that North American pharmaceutical and biotechnology companies and life science research institutions are planning a $14.7-billion building boom, with more than 320 active capital projects.

Cambrex (East Rutherford, NJ) to produce Geron (Menlo Park, CA) telomerase anti-cancer vaccine. Generex (Toronto, ON.) files IND for synthetic vaccine to stimulate cell-mediated immunity to avian influenza. Dow Agrosciences (Indianapolis, IN) wins USDA approval for veterinary vaccine, the first manufactured via plant cell culture. G-8 nations pledge billons for vaccine production.

The Pharmaceutical Industry has been slow in adopting radio frequency technology (RFID) to help control diversion and counterfeiting, according to a recent study by ABI Research (Oyster Bay, NY, www.abiresearch.com). In fact, only 10 drug products are expected to be shipped with RFID tags or smart chips embedded in the labeling in the coming year.

The US Food and Drug Administration’s $1.95-billion budget request for the 2007 fiscal increases spending on high-priority programs by $70 million, and cuts support for some existing programs by $52 million.

Merck wins approval for rotavirus vaccine as Sanofi ships investigational H5N1 vaccine to NIH and CDC explores new flue diagnostics and novel vaccine-production technology.

ASTM International’s (West Conshohocken, PA, www.astm.org) Committee E55 on Pharmaceutical Application of Process Analytical Technology has established a subcommittee “E55.03 on General Pharmaceutical Standards,” to focus on issues relating to quality within a chemistry, manufacturing, and control framework.

Eggs from Transgenic Hens Express Interferon Beta-1a Protein

Preliminary results from a clinical trial of Sanofi Pasteur's (Lyon, France, www.sanofi.com) H5N1 prepandemic influenza vaccine indicate the vaccine is safe and was well-tolerated in 300 healthy volunteers. This study is the first trial of an H5N1 prepandemic influenza vaccine candidate that compared vaccines with and without adjuvants.

Cell-Grown Vaccine Protects Against Avian Flu Virus

Non-Injectable Insulins Win Approval: Pfizer in the US, Generex in South America
The European Science Foundation (ESF) has published the conclusions of its 2-year Forward Look Study on Nanomedicine. Defined as a billionth of a metre, a nanometer is 1000 times smaller than the width of a human hair. Nanomedicine uses nanoscale technology to diagnose and treat disease. The scope of the study included defining the field of nanomedicine, reviewing what has been achieved so far, determining Europe's strengths and weaknesses, and drawing up plans to ensure continued growth.

Small- and medium-sized enterprises (SMEs) will gain administrative and procedural assistance from the EMEA. The agency's SME office was launched in December 2005 and follows the new Commission Regulation, which aims to promote the development of medicinal products in SMEs.

FDA Unveils New Prescription Drug Information Format

AAIPharma Moves Ahead with Reorganization Plan

FDA Eases Phase I Manufacturing Requirements

Warning: Brazilian Diet Pills Found to Contain Active Drug Ingredients

Pfizer Combats Counterfeiters with RFID

FDA Issues Dispute–Resolution Guidance

Novartis Considers Acquiring Berna Biotech

Schering-Plough Meets FDA Deadline for CGMP Improvements

Sanofi Pasteur and BD Team Up to Produce Microneedle Vaccines

Hubert J.P. Schoemaker Dead at 55
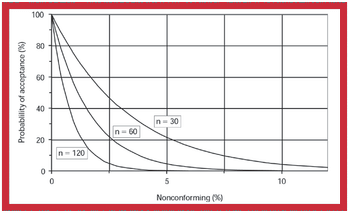
The benefits of zero tolerance as a test criterion have been oversold. A critical examination of zero tolerance reveals that many of the supposed benefits are not attainable. More important, inappropriate application of this criterion can have a deleterious effect on the assessment, control, and improvement of the quality of pharmaceutical products.