Pharmaceutical Technology Editors
Articles by Pharmaceutical Technology Editors

The US Food and Drug Administration?s Counterfeit Drug Task Force (Rockville, MD, www.fda.gov) is recommending regulatory actions and the implementation of new technologies for reducing the risk of counterfeit drugs entering the United States. The group has followed up on its original 2004 report, in which it outlined the framework for protecting the public from counterfeit medicines, and an updated 2005 report with a third document encouraging electronic pedigrees, improved traceability in the drug supply chain, and the adoption of radio-frequency identification (RFID) tools.

The US Department of Health and Human Services (HHS, Washington, DC) awarded biotech company Cangene (Winnipeg, MB, Canada) a $362-million Project Bioshield supply contract for 200,000 doses of botulinum toxin immune globulin (heptavalent botulism antitoxin).

The Parenteral Drug Association (PDA, Bethesda, MD) finished the second stage of GMP training for representatives from the Republic of Kazakhstan?s Ministry of Health.

Abemarle, Aceto, Nastech Pharmaceutical Company, Novartis, Novo Nordisk, PerkinElmer, Inc.

RNAi therapeutics company SR Pharma plc (London, UK) has developed a process that allows it proprietary liposomal-based siRNA formulations ?AtuRNAi? drugs to be stored at room temperature and reconstituted in one step.

Recombinomics (Pittsburgh, PA) is again urging the World Health Organization to fully release all H5N1 avian influenza sequences, claiming their release would improve the selection of vaccines by helping scientists to identify the origin of the isolates and predict sequence changes.

Solutia Inc. (St. Louis, MO) announced that its subsidiary, Solutia Europe SA/NV (SESA), had reached a definitive agreement to sell its pharmaceutical services business, which comprises CarboGen AG and AMCIS AG. Under the terms of the agreement, Dishman Pharmaceuticals and Chemicals Ltd. (Ahmedabad, India) will purchase 100 percent of CarboGen and AMCIS stock and related assets for $74.5 million, subject to a working capital adjustment.

Noting several violations in current good manufacturing practice regulations, the US Food and Drug Administration has issued a Warning Letter to Wyeth Pharmaceuticals?s (Collegville, PA) manufacturing facility in Puerto Rico.

BASF, Bayer, NovaDel, Parexel, Schering

The US Food and Drug Administration (Rockville, MD) is withdrawing seven guidance documents "because some of the principles in these guidances are inconsistent with the agency's initiative, Pharmaceutical Current Good Manufacturing Practices (CGMPs) for the 21st Century (CGMP Initiative) .

On May 26, AstraZeneca (Wilmington, DE) announced it would invest $100 million in research and development in China over the next three years.
Pharmaceutical Science & Technology News

Abraxis BioScience Inc., Bristol-Myers Squibb Company, Cambrex Corporation, Sigma-Aldrich Corporation, SkyePharma

The CDER and CBER have released a new "Guidance for Industry: Q8 Pharmaceutical Development," outlining what drug manufacturers should include in the Pharmaceutical Development section of International Council on Harmonization (ICH) Common Technical Document (CTD) submissions.

Spectrum Laboratory Products (Gardena, CA) recalled its active pharmaceutical ingredient, tacrolimus, after learning that some lots of the ingredient are subpotent. At least one injury has been reported.

Research and Markets (Dublin, Ireland) has released ?Annual Investment Analysis Report of the Chinese Pharmaceutical Logistics Industry, 2005-2006.? The report provides analysis of the pharmaceutical logistics characteristics, conditions, pharmaceutical market and pharmaceutical retail dynamics.

The US Food and Drug Administration (Rockville, MD) seeks volunteers to take part in a pilot project to test a Health Level 7 (HL7) data-interchange standard for submitting product stability data.

Boehringer Ingelheim, Crucell, Eisai, Laureate Pharma, Pall Corp., Tunnell Consulting

Roche (Basel, Switzerland) has entered into a nonexclusive agreement with Aspen Pharmacare (Port Elizabeth, South Africa) to speed up production and supply of a generic version of oseltamivir ("Tamiflu") for Africa.
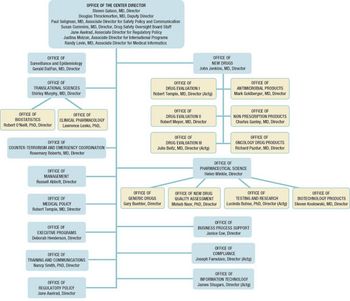
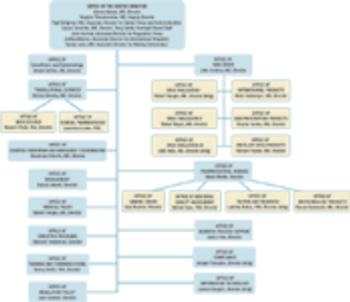
The US Food and Drug Administration?s Center for Drug Evaluation and Research (CDER, Rockville, MD) formally put into action its reorganization plan, effective May 15.

Seven automation suppliers have joined with users on the Instrumentation, Systems, and Automation Society (ISA, Research Triangle Park, NC) "Wireless Standards for Automation (SP100)" committee to develop wireless network structures for manufacturing plants.

The US Department of Health and Human Services (HHS, Washington, DC) will distribute more than $1 billion in contracts to accelerate the development of cell-based production technologies for influenza vaccines.

Johnson & Johnson, Mesa Labs, Mar Cor, FDA, Andrx

On April 28, the US Food and Drug Administration's Center for Drug Evaluation and Research (Rockville, MD) issued a Warning Letter to Pliva Hrvatska d.o.o., a subsidiary of Pliva d.d. (Zagreb, Croatia).

On May 8, Thermo Electron Corporation (Waltham, MA) and Fisher Scientific International Inc. (Hampton, NH) announced they would merge in a tax-free, stock-for-stock exchange.

The US Food and Drug Administration?s (FDA, Rockville, MD, www.fda.gov) Center for Drug Evaluation and Research (CDER) is studying its own Advisory Committee Meeting system to identify best practices for this process.

The US Food and Drug Administration?s (Rockville, MD) Center for Drug Evaluation and Research (CDER) received 637 commercial investigational new drug (IND) applications in 2005, a 20-year high.

In a May 2 Federal Register notice (1), the US Food and Drug Administration withdrew its Jan. 17 direct final rule, "Current Good Manufacturing Practice Regulation and Investigational New Drugs" (2), which would have exempted manufacturing of drugs for Phase I clinical trials from most provisions of 21 CFR 211.

Cobra Biomanufacturing, Avecia, Dynavax Technologies, BASF, Codexis

Sigma-Aldrich Corporation (St. Louis, MO) has acquired Iropharm, Honeywell International's custom chemical synthesis business in Arklow, Ireland. Terms of the cash purchase were not disclosed.



