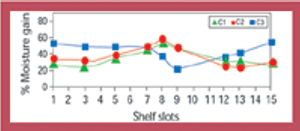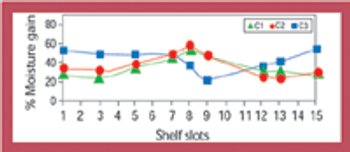
Stability data that do not follow the expected trend in comparison with other stability batches or previous results collected during a stability study are considered out-of-trend (OOT) results. OOT stability results recently have gained the attention of regulatory agencies and as a result, the approach for identifying and investigating OOT results has become a topic of increased discussion. One example is a 2003 article by the PhRMA Chemistry, Manufacturing, and Controls Statistics and Stability Expert Teams, which was intended to initiate dialogue on how to address OOT results (1).