
Grace McNally, in the Office of Compliance at FDA's CDER, discusses the role of QbD and pharmaceutical quality systems as it relates to contract manufacturing.

Grace McNally, in the Office of Compliance at FDA's CDER, discusses the role of QbD and pharmaceutical quality systems as it relates to contract manufacturing.
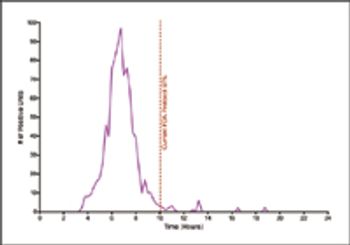
This study used biological indicators containing Geobacillus stearothermophilus spores and a new technology to continuously monitor incubated BIs and record nonsterile results.
The authors sought to prepare a topical formulation of berberine hydrochloride for the effective and controlled management of inflammation and skin infections.

To move from R&D to breakthrough drugs, biotech companies need policies that support innovation.

Globalization and reform initiatves will shape pharma production, pricing, and pipelines in 2010.

Some predictions for contract services in 2010.

Is Brazil's pharmaceutical industry following the consolidation trend?

A recent deal could chart new paths in drug delivery and in the administration of vaccines.
Growth in emerging markets and across the generic-drug sector shifts the global demand and supply of active pharmaceutical ingredients.
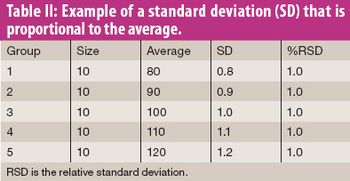
When to use percent relative standard deviation-and how to do so correctly.

Editors' Picks of Pharmaceutical Science & Technology Innovations

A comprehensive book about mass transfer benefits from the author's personal touch.

Team leaders in FDA's Office of Generic Drugs provide an overview.

Plus, novel dosage forms and emerging trends.
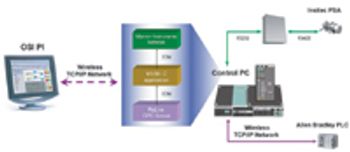
The authors describe the implementation of an on-line particle-size analyzer on an active pharmaceutical ingredient milling operation at a commercial site.
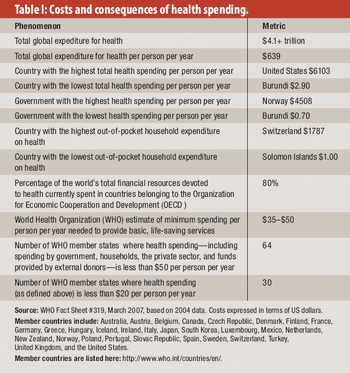
From healthcare to corruption to life expectancy, here's what we can learn from the past decade.

Directors and staff miss the mark when it comes to following procedures.

Quality by Design, Deficiencies in Generic Drug Applications, API Growth, Topical Berberine-Hydrochloride Products